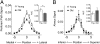Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo - PubMed (original) (raw)
Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo
Michael A Yassa et al. Proc Natl Acad Sci U S A. 2010.
Abstract
The perforant path (PP) undergoes synaptic changes in the course of aging and dementia. Previous studies attempting to assess the integrity of the PP in humans using diffusion tensor imaging (DTI) were limited by low resolution and the inability to identify PP fibers specifically. Here we present an application of DTI at ultrahigh submillimeter resolution that has allowed us to successfully identify diffusion signals unique to the PP and compare the intensity of these signals in a sample of young adults and older adults. We report direct evidence of age-related PP degradation in humans in vivo. We find no evidence of such loss in a control pathway, the alveus, suggesting that these findings are not evidence for a global decline. We also find no evidence for specific entorhinal gray matter atrophy. The extent of PP degradation correlated with performance on a word-list learning task sensitive to hippocampal deficits. We also show evidence for gray matter diffusion signals consistent with pyramidal dendrite orientation in the hippocampus and cerebral cortex. Ultrahigh-resolution microstructural DTI is a unique biomarker that can be used in combination with traditional structural and functional neuroimaging methods to enhance detection of Alzheimer disease in its earliest stages, test the effectiveness of new therapies, and monitor disease progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
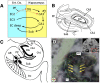
Fig. 1.
(A) Circuit figure of hippocampal connectivity showing the PP. EC2, entorhinal cortex layer II; EC3, entorhinal cortex layer III; EC deep, entorhinal cortex deep layers; DG, dentate gyrus; Sub, subiculum. The broken red line emphasizes that this pathway is degraded with aging. (B) A retrograde tracer diagram of the PP [Reproduced with permission from ref. (Copyright John Wiley and Sons, 1991)]. (C) Schematized illustration of PP connectivity in the hippocampus [Adapted with permission from ref. (Copyright 1986, John Wiley and Sons)]. (D) Single hippocampal DTI slice clearly showing the tensor orientation of the PP fibers (the slice used here for tensor visualization is approximately twice as thick as the slices used in the main quantification analyses). (Inset) Structural MRI scan showing the position of this slice and the magnified location. The tensor map is overlaid on an FA map that is modulated by the grayscale anatomical image.
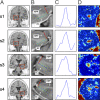
Fig. 2.
Sample measurements from two young (s1, s2) and two old (s3, s4) subjects (two from each hemisphere). (A) Coronal slice anatomical location where the measurement was conducted. Red boxes indicate the approximate location of the zoomed-in view in B. This view is a close-up of the anatomy with the blue dots defining a line parallel to the entorhinal cortex sheet (green dots are 1 voxel in each direction) and the red line is the direction perpendicular to it (the actual PP direction). The hippocampus (HIPP) and the entorhinal cortex (EC) are labeled on each slice. (C) One-dimensional plots of the PPproj value clearly show a peak consistent with the position of the PP. (D) The 2D plots of the PPproj value in the entire volume shown in B are color-coded so “hot” colors (red/yellow) are greater PPproj values (i.e., larger diffusion signal along the PP direction) and “cold” colors (blue/purple) are lower PPproj values (i.e., smaller diffusion signal along the PP direction). The hot spots in the middle of each spot represent the PP on that particular slice.
Fig. 3.
(A) Average PP signal curves from young and old subjects show a significant group difference between young and old subjects in AUC. (B) Average alveus signal curves from young and old subjects show no significant difference between groups in AUC.
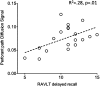
Fig. 4.
Significant correlation between PP AUC and delayed recall performance on the RAVLT in older adults.
Similar articles
- Examining the gateway to the limbic system with diffusion tensor imaging: the perforant pathway in dementia.
Kalus P, Slotboom J, Gallinat J, Mahlberg R, Cattapan-Ludewig K, Wiest R, Nyffeler T, Buri C, Federspiel A, Kunz D, Schroth G, Kiefer C. Kalus P, et al. Neuroimage. 2006 Apr 15;30(3):713-20. doi: 10.1016/j.neuroimage.2005.10.035. Epub 2005 Dec 6. Neuroimage. 2006. PMID: 16337815 - Mnemonic discrimination relates to perforant path integrity: An ultra-high resolution diffusion tensor imaging study.
Bennett IJ, Stark CE. Bennett IJ, et al. Neurobiol Learn Mem. 2016 Mar;129:107-12. doi: 10.1016/j.nlm.2015.06.014. Epub 2015 Jul 4. Neurobiol Learn Mem. 2016. PMID: 26149893 Free PMC article. - Reduced structural connectivity of the medial temporal lobe including the perforant path is associated with aging and verbal memory impairment.
Granger SJ, Colon-Perez L, Larson MS, Bennett IJ, Phelan M, Keator DB, Janecek JT, Sathishkumar MT, Smith AP, McMillan L, Greenia D, Corrada MM, Kawas CH, Yassa MA. Granger SJ, et al. Neurobiol Aging. 2023 Jan;121:119-128. doi: 10.1016/j.neurobiolaging.2022.10.012. Epub 2022 Nov 9. Neurobiol Aging. 2023. PMID: 36434930 Free PMC article. - Diffusion tensor imaging in Alzheimer's disease and mild cognitive impairment.
Stebbins GT, Murphy CM. Stebbins GT, et al. Behav Neurol. 2009;21(1):39-49. doi: 10.3233/BEN-2009-0234. Behav Neurol. 2009. PMID: 19847044 Free PMC article. Review. - Diffusion tensor imaging of the hippocampus in MCI and early Alzheimer's disease.
Fellgiebel A, Yakushev I. Fellgiebel A, et al. J Alzheimers Dis. 2011;26 Suppl 3:257-62. doi: 10.3233/JAD-2011-0001. J Alzheimers Dis. 2011. PMID: 21971465 Review.
Cited by
- Cerebral hyperactivation across the Alzheimer's disease pathological cascade.
Corriveau-Lecavalier N, Adams JN, Fischer L, Molloy EN, Maass A. Corriveau-Lecavalier N, et al. Brain Commun. 2024 Oct 25;6(6):fcae376. doi: 10.1093/braincomms/fcae376. eCollection 2024. Brain Commun. 2024. PMID: 39513091 Free PMC article. Review. - Entorhinal cortex-hippocampal circuit connectivity in health and disease.
Hernández-Frausto M, Vivar C. Hernández-Frausto M, et al. Front Hum Neurosci. 2024 Sep 20;18:1448791. doi: 10.3389/fnhum.2024.1448791. eCollection 2024. Front Hum Neurosci. 2024. PMID: 39372192 Free PMC article. Review. - An aging-sensitive compensatory secretory phospholipase that confers neuroprotection and cognitive resilience.
Vicidomini C, Goode TD, McAvoy KM, Yu R, Beveridge CH, Iyer SN, Victor MB, Leary N, Evans L, Steinbaugh MJ, Lai ZW, Lyon MC, Silvestre MRFS, Bonilla G, Sadreyev RI, Walther TC, Sui SH, Saido T, Yamamoto K, Murakami M, Tsai LH, Chopra G, Sahay A. Vicidomini C, et al. bioRxiv [Preprint]. 2024 Sep 3:2024.07.26.605338. doi: 10.1101/2024.07.26.605338. bioRxiv. 2024. PMID: 39211220 Free PMC article. Preprint. - Increasing NPYergic transmission in the hippocampus rescues aging-related deficits of long-term potentiation in the mouse dentate gyrus.
Klinger K, Del Ángel M, Çalışkan G, Stork O. Klinger K, et al. Front Aging Neurosci. 2023 Nov 9;15:1283581. doi: 10.3389/fnagi.2023.1283581. eCollection 2023. Front Aging Neurosci. 2023. PMID: 38020778 Free PMC article. - Age-related differences in hippocampal subfield volumes across the human lifespan: A meta-analysis.
Homayouni R, Canada KL, Saifullah S, Foster DJ, Thill C, Raz N, Daugherty AM, Ofen N. Homayouni R, et al. Hippocampus. 2023 Dec;33(12):1292-1315. doi: 10.1002/hipo.23582. Epub 2023 Oct 26. Hippocampus. 2023. PMID: 37881160 Free PMC article.
References
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. - PubMed
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. - PubMed
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous