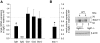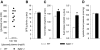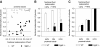SGLT2 mediates glucose reabsorption in the early proximal tubule - PubMed (original) (raw)
SGLT2 mediates glucose reabsorption in the early proximal tubule
Volker Vallon et al. J Am Soc Nephrol. 2011 Jan.
Abstract
Mutations in the gene encoding for the Na(+)-glucose co-transporter SGLT2 (SLC5A2) associate with familial renal glucosuria, but the role of SGLT2 in the kidney is incompletely understood. Here, we determined the localization of SGLT2 in the mouse kidney and generated and characterized SGLT2-deficient mice. In wild-type (WT) mice, immunohistochemistry localized SGLT2 to the brush border membrane of the early proximal tubule. Sglt2(-/-) mice had glucosuria, polyuria, and increased food and fluid intake without differences in plasma glucose concentrations, GFR, or urinary excretion of other proximal tubular substrates (including amino acids) compared with WT mice. SGLT2 deficiency did not associate with volume depletion, suggested by similar body weight, BP, and hematocrit; however, plasma renin concentrations were modestly higher and plasma aldosterone levels were lower in Sglt2(-/-) mice. Whole-kidney clearance studies showed that fractional glucose reabsorption was significantly lower in Sglt2(-/-) mice compared with WT mice and varied in Sglt2(-/-) mice between 10 and 60%, inversely with the amount of filtered glucose. Free-flow micropuncture revealed that for early proximal collections, 78 ± 6% of the filtered glucose was reabsorbed in WT mice compared with no reabsorption in Sglt2(-/-) mice. For late proximal collections, fractional glucose reabsorption was 93 ± 1% in WT and 21 ± 6% in Sglt2(-/-) mice, respectively. These results demonstrate that SGLT2 mediates glucose reabsorption in the early proximal tubule and most of the glucose reabsorption by the kidney, overall. This mouse model mimics and explains the glucosuric phenotype of individuals carrying SLC5A2 mutations.
Figures
Figure 1.
Generation of transgenic _Sglt2_−/− mice. (A) Targeting strategy used to disrupt the Slc5a2 locus. Homologous recombination (represented by X) between the targeting vector and the Slc5a2 gene results in the replacement of exons 1 through 5 with the selection cassette. (B) Southern hybridization indicating proper gene targeting in four ES cell clones. Clone 4 is transmitted through the germline; control represents untransfected ES cell DNA. (C) PCR amplification of tail-snip DNA from _Sglt2_−/−, WT (+/+), and heterozygous (+/−) mice. Separate PCRs are performed for the WT allele (left lane) and the targeted allele (right lane) for each sample.
Figure 2.
Renal expression of Glut1, Glut2, and Glut12 is unaltered but the expression of Sglt1 is reduced in _Sglt2_−/− mice. (A) Renal mRNA expression of Glut1, Glut2, and Glut12 are unchanged whereas Sglt1 is reduced in _Sglt2_−/− relative to WT mice. Real-time PCR confirms the knockout of Sglt2 in kidney of _Sglt2_−/− mice. ND, not detectable. n = 5 per genotype. *P < 0.01 versus WT mice. (B) Western blotting shows reduced renal SGLT1 protein expression (related to β-actin) in _Sglt2_−/− versus WT mice (n = 4 per genotype). *P < 0.05 versus WT mice. Gene knockout of Sglt1 in mice (_Sglt1_−/−) confirms the specificity of the signal.
Figure 3.
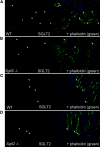
Expression of SGLT2 protein in the apical brush border membrane of the early proximal convoluted tubule. (A through D) Renal cortical sections (A and B) and outer medullary sections (C and D) are shown for WT and _Sglt2_−/− mice. Left pictures show staining with SGLT2 Ab (red fluorescence); right pictures show additional co-staining with phalloidin (green fluorescence) to label filamentous actin of the proximal tubular brush border. Nuclei are stained with the marker DAPI (blue fluorescence). (A and B) The SGLT2 Ab provides specific and selective staining of the apical brush border membrane of the early proximal convoluted tubules. The staining for SGLT2 begins where the proximal tubule initiates from Bowman space (*) and ends along the proximal convoluted tubule (arrowhead). Further downstream sections of the proximal convoluted tubule (identified by reduced height of brush border) do not stain for SGLT2 (arrows). (C and D) No specific SGLT2 signal is detected in the proximal straight tubules of the outer medulla. Arrows mark the distal ends of proximal straight tubules.
Figure 4.
Urinary glucose concentration is enhanced in _Sglt2_−/− mice despite normal blood glucose levels and GFR: Studies in awake mice. (A) Glucose measured in spontaneous urine collections and subsequent blood collections from tail vein. (B) GFR determined by plasma FITC-inulin kinetics. (C) Food and fluid intake assessed in regular cages. (D) BP measurements using a tail-cuff system in trained awake mice (n = 9 to 10 per group). *P < 0.05 versus WT mice.
Figure 5.
Renal excretion of glucose in _Sglt2_−/− is a function of the amounts of glucose filtered: Clearance studies under anesthesia (n = 9 to 10 per group).
Figure 6.
Reabsorption of glucose is absent in the early proximal tubule of _Sglt2_−/− mice: Micropuncture studies under anesthesia. (A) Free-flow collections of tubular fluid are performed along accessible proximal tubules at the kidney surface to establish a profile for FR-glucose versus FR-fluid. (B and C) Mean FR-glucose (B) and fractional reabsorption of chloride (C) for early (FR-fluid <40%) and late (FR-fluid ≥40%) proximal tubular collections and up to the urine (n = 18 to 23 nephrons in four to five mice). *P < 0.001 versus WT mice.
Comment in
- Risks and benefits of Sweet Pee.
Lang F. Lang F. J Am Soc Nephrol. 2011 Jan;22(1):2-5. doi: 10.1681/ASN.2010091006. Epub 2010 Dec 16. J Am Soc Nephrol. 2011. PMID: 21164030 No abstract available.
Similar articles
- Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia.
Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, Thomson SC, Koepsell H, Vallon V. Rieg T, et al. Am J Physiol Renal Physiol. 2014 Jan;306(2):F188-93. doi: 10.1152/ajprenal.00518.2013. Epub 2013 Nov 13. Am J Physiol Renal Physiol. 2014. PMID: 24226519 Free PMC article. - Organic anion transporter OAT3 enhances the glucosuric effect of the SGLT2 inhibitor empagliflozin.
Fu Y, Breljak D, Onishi A, Batz F, Patel R, Huang W, Song P, Freeman B, Mayoux E, Koepsell H, Anzai N, Nigam SK, Sabolic I, Vallon V. Fu Y, et al. Am J Physiol Renal Physiol. 2018 Aug 1;315(2):F386-F394. doi: 10.1152/ajprenal.00503.2017. Epub 2018 Feb 7. Am J Physiol Renal Physiol. 2018. PMID: 29412698 Free PMC article. - De novo expression of sodium-glucose cotransporter SGLT2 in Bowman's capsule coincides with replacement of parietal epithelial cell layer with proximal tubule-like epithelium.
Tabatabai NM, North PE, Regner KR, Kumar SN, Duris CB, Blodgett AB. Tabatabai NM, et al. J Membr Biol. 2014 Aug;247(8):675-83. doi: 10.1007/s00232-014-9686-4. Epub 2014 Jun 7. J Membr Biol. 2014. PMID: 24906870 Free PMC article. - Probing SGLT2 as a therapeutic target for diabetes: basic physiology and consequences.
Gallo LA, Wright EM, Vallon V. Gallo LA, et al. Diab Vasc Dis Res. 2015 Mar;12(2):78-89. doi: 10.1177/1479164114561992. Epub 2015 Jan 23. Diab Vasc Dis Res. 2015. PMID: 25616707 Free PMC article. Review. - Targeting renal glucose reabsorption to treat hyperglycaemia: the pleiotropic effects of SGLT2 inhibition.
Vallon V, Thomson SC. Vallon V, et al. Diabetologia. 2017 Feb;60(2):215-225. doi: 10.1007/s00125-016-4157-3. Epub 2016 Nov 22. Diabetologia. 2017. PMID: 27878313 Free PMC article. Review.
Cited by
- In nondiabetic C57BL/6J mice, canagliflozin affects the skeleton in a sex- and age-dependent manner.
Chlebek C, McAndrews C, Costa SN, DeMambro VE, Yakar S, Rosen CJ. Chlebek C, et al. JBMR Plus. 2024 Oct 10;8(12):ziae128. doi: 10.1093/jbmrpl/ziae128. eCollection 2024 Dec. JBMR Plus. 2024. PMID: 39502898 Free PMC article. - Gene therapy of Dent disease type 1 in newborn ClC-5 null mice for sustained transgene expression and gene therapy effects.
Lyu P, Yadav MK, Yoo KW, Jiang C, Li Q, Atala A, Lu B. Lyu P, et al. Gene Ther. 2024 Sep 25. doi: 10.1038/s41434-024-00490-w. Online ahead of print. Gene Ther. 2024. PMID: 39322766 - Exploring Varied Treatment Strategies for Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD).
Elshaer A, Chascsa DMH, Lizaola-Mayo BC. Elshaer A, et al. Life (Basel). 2024 Jul 3;14(7):844. doi: 10.3390/life14070844. Life (Basel). 2024. PMID: 39063598 Free PMC article. Review. - Actin cytoskeleton and associated myosin motors within the renal epithelium.
Busselman BW, Ratnayake I, Terasaki MR, Thakkar VP, Ilyas A, Otterpohl KL, Zimmerman JL, Chandrasekar I. Busselman BW, et al. Am J Physiol Renal Physiol. 2024 Oct 1;327(4):F553-F565. doi: 10.1152/ajprenal.00078.2024. Epub 2024 Jul 25. Am J Physiol Renal Physiol. 2024. PMID: 39052845 Review.
References
- Wright EM: Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol 280: F10–F18, 2001 - PubMed
- Horiba N, Masuda S, Takeuchi A, Takeuchi D, Okuda M, Inui K: Cloning and characterization of a novel Na+-dependent glucose transporter (NaGLT1) in rat kidney. J Biol Chem 278: 14669–14676, 2003 - PubMed
- Wright EM, Hirayama BA, Loo DF: Active sugar transport in health and disease. J Intern Med 261: 32–43, 2007 - PubMed
- Brown GK: Glucose transporters: Structure, function and consequences of deficiency. J Inherit Metab Dis 23: 237–246, 2000 - PubMed
- Wright EM, Turk E: The sodium/glucose cotransport family SLC5. Pflugers Arch 447: 510–518, 2004 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 DK028602/DK/NIDDK NIH HHS/United States
- P30DK079337/DK/NIDDK NIH HHS/United States
- DK28602/DK/NIDDK NIH HHS/United States
- R01 DK056248/DK/NIDDK NIH HHS/United States
- P30 DK079337/DK/NIDDK NIH HHS/United States
- DK56248/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases