CB1 receptor antagonism impairs the induction of epileptiform activity by group I metabotropic glutamate receptor activation - PubMed (original) (raw)
CB1 receptor antagonism impairs the induction of epileptiform activity by group I metabotropic glutamate receptor activation
Linda Karr et al. Epilepsia. 2010 Jul.
Abstract
Exposure to the group I metabotropic glutamate receptor (mGluR) agonist dihydroxy phenylglycine (DHPG) induces epileptiform activity in the CA3 region of the hippocampus that persists following washout of DHPG. DHPG also can cause long-term depression of synaptic transmission, and at some synapses this may be mediated by endocannabinoids. We evaluated whether the selective cannabinoid type 1 (CB1) receptor antagonists SR 141716 or AM 251 could modify induction of epileptiform activity produced by DHPG exposure. The induction of epileptiform activity by DHPG exposure was significantly reduced by CB1 receptor antagonists, SR 141716 or AM 251. Minimal effects on epileptiform activity were noted once the activity had been induced. In control slices, exposure to DHPG for 30 min produced long-term depression (LTD) of synaptic transmission, on average about a 70% reduction in slope of the field excitatory postsynaptic potential (EPSP). When slices were exposed to both DHPG and SR 141716 (3 microm), LTD did not occur and the population EPSP remained at control values or greater. These results suggest that CB1 receptors mediate some of DHPG effects that result in persistent epileptiform activity, and antagonism of CB1 receptors has antiepileptogenic properties. Paradoxically DHPG also caused LTD of excitatory synaptic transmission in the CA3 region and CB1 receptor antagonism prevents the depression. We hypothesize that the ictal activity induced by DHPG requires depression of synaptic strength and CB1 receptor antagonism prevents this depression and the induction of ictal activity.
Conflict of interest statement
Conflict of Interest Statement: None of the authors have any conflicts of interest and the paper conforms to the ethical guidelines of Epilepsia for publication.
Figures
Figure 1
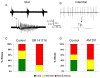
Induction of epileptiform activity by DHPG was suppressed by CB1 antagonists. Slices exposed to DHPG resulted in ictal patterns of activity (A), interictal activity (B) or no spontaneously occurring network activity (not bursting). Ictal activity consisted of recurrent population discharges that occurred for longer than 2 s at greater than 2 Hz (A, scale bar with 0.4 mV and time scale of 20 s for top trace and 4 s for bottom trace). Interictal activity consisted of recurrent brief (less than 500 ms) discharges (B, scale bar of 2 mV and time scale of 20 s and 50 ms). C: Following exposure to DHPG and after a 1 hour wash period, control slices showed ictal activity in 44% of slices (green) with 32% demonstrating interictal activity (yellow), and 24 % not bursting (red) (n = 26). When SR 141716 (3 μM) was applied prior to and with the DHPG no slices showed ictal discharges and 23% had interictal discharges (p < 0.001). D: Similar findings occurred with AM 521 (5 μM) with only 5% of slices demonstrating ictal discharges (n = 37) compared to 41% of control slices (n = 39, p =0.001).
Figure 2
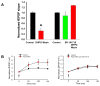
A: SR 141716 (3 μM) blocked long term depression that followed DHPG exposure. In control slices a 30 minute exposure to DHPG followed by a 60 minute wash to aCSF resulted in a significant reduction in the fEPSP slope (n = 9, p < 0.001 *) whereas SR141716 prevented the long term depression associated with DHPG exposure (n =7). SR 141716 depressed the fEPSP by about 11% (green bar, not significant). B: The induction of long term depression was associated with enhanced facilitation of the fEPSP compared to the control condition. Stimulation in stratum radiatum was given at 20 Hz (4 pulses) at the arrow and the population EPSP (pEPSP) amplitude was normalized to the first response. Enhanced facilitation was not noted when slices were exposed to DHPG in the presence of SR 141716.
Similar articles
- Endocannabinoid- and mGluR5-dependent short-term synaptic depression in an isolated neuron/bouton preparation from the hippocampal CA1 region.
Sheinin A, Talani G, Davis MI, Lovinger DM. Sheinin A, et al. J Neurophysiol. 2008 Aug;100(2):1041-52. doi: 10.1152/jn.90226.2008. Epub 2008 May 21. J Neurophysiol. 2008. PMID: 18497350 Free PMC article. - Postsynaptic induction and presynaptic expression of group 1 mGluR-dependent LTD in the hippocampal CA1 region.
Watabe AM, Carlisle HJ, O'Dell TJ. Watabe AM, et al. J Neurophysiol. 2002 Mar;87(3):1395-403. doi: 10.1152/jn.00723.2001. J Neurophysiol. 2002. PMID: 11877514 - Dual modulation of excitatory synaptic transmission by agonists at group I metabotropic glutamate receptors in the rat spinal dorsal horn.
Zhong J, Gerber G, Kojić L, Randić M. Zhong J, et al. Brain Res. 2000 Dec 29;887(2):359-77. doi: 10.1016/s0006-8993(00)03066-3. Brain Res. 2000. PMID: 11134626 - Endocannabinoids contribute to short-term but not long-term mGluR-induced depression in the hippocampus.
Rouach N, Nicoll RA. Rouach N, et al. Eur J Neurosci. 2003 Aug;18(4):1017-20. doi: 10.1046/j.1460-9568.2003.02823.x. Eur J Neurosci. 2003. PMID: 12925027 - Weeding out bad waves: towards selective cannabinoid circuit control in epilepsy.
Soltesz I, Alger BE, Kano M, Lee SH, Lovinger DM, Ohno-Shosaku T, Watanabe M. Soltesz I, et al. Nat Rev Neurosci. 2015 May;16(5):264-77. doi: 10.1038/nrn3937. Nat Rev Neurosci. 2015. PMID: 25891509 Free PMC article. Review.
Cited by
- CUMYL-4CN-BINACA Is an Efficacious and Potent Pro-Convulsant Synthetic Cannabinoid Receptor Agonist.
Kevin RC, Anderson L, McGregor IS, Boyd R, Manning JJ, Glass M, Connor M, Banister SD. Kevin RC, et al. Front Pharmacol. 2019 May 29;10:595. doi: 10.3389/fphar.2019.00595. eCollection 2019. Front Pharmacol. 2019. PMID: 31191320 Free PMC article. - Metabotropic Glutamate Receptors and Interacting Proteins in Epileptogenesis.
Qian F, Tang FR. Qian F, et al. Curr Neuropharmacol. 2016;14(5):551-62. doi: 10.2174/1570159x14666160331142228. Curr Neuropharmacol. 2016. PMID: 27030135 Free PMC article. Review. - Fragile X syndrome and targeted treatment trials.
Hagerman R, Lauterborn J, Au J, Berry-Kravis E. Hagerman R, et al. Results Probl Cell Differ. 2012;54:297-335. doi: 10.1007/978-3-642-21649-7_17. Results Probl Cell Differ. 2012. PMID: 22009360 Free PMC article. Review.
References
- During MJ, Spencer DD. Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. Lancet. 1993;341:1607–1610. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous