Macrophages and immunologic inflammation of the kidney - PubMed (original) (raw)
Review
Macrophages and immunologic inflammation of the kidney
Jeremy S Duffield. Semin Nephrol. 2010 May.
Abstract
Monocyte-derived tissue effector cells, macrophages, are present in large numbers in all forms of kidney disease with inflammation. Their roles in inflammation and the molecular effectors of macrophage function have been difficult to decipher. With the advent of modern genetic tools and mouse models of human disease, great insight into monocyte/macrophage biology has been forthcoming. This review places macrophage study in its historical context, defines immunologic diseases of the kidney, broadens its definition to encompass current thinking of the immune response to kidney injury, highlights key advances of the study of monocyte/macrophages in kidney diseases, and identifies new therapeutic pathways and targets that hinge around macrophage function. This article advances the case that targeting macrophage activation and phenotype is leading to new therapies in the treatment of many acute and chronic kidney diseases.
Figures
Figure 1. Macrophages are present in all forms of kidney disease with inflammation
Photomicrographs of (A) human glomerulonephritis labeled with antibodies (brown) against the macrophage marker CD68 (T = tubule, G = glomerulus). (B) mouse model of chronic kidney disease showing macrophages (brown) labeled with the F4/80 antibody against EMR1. (C-D) Graph showing the proportion kidney cells that are macrophages in two mouse models of kidney disease. Note that macrophages are present in chronic disease (C) but also in repair after injury (D). Marker = 100µm
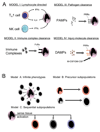
Figure 2. Schematics demonstrating likely mechanisms of
(A) macrophage activation in vivo. In addition to pathogens (Model III), lymphocytes, immune complexes and molecules released from damaged tissue all have the capacity to activate macrophages. (B) Three models proposed to explain macrophage heterogeneity in tissue inflammation. In model A an infinite number of Mϕ phenotypes can occur, in model B there are subsets of monocytes that are preprogrammed with a stereotyped response and in model C monocytes differentiate into a restricted number of phenotypes depending on both the tissue environment and an activating stimulus.
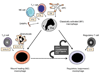
Figure 3. Subpopulations of inflammatory macrophages in vivo
Schematic showing three different types of inflammatory macrophage, and factors that regulate their activation/differentiation in sterile inflammation in vivo. Although cell-derived and tissue-derived factors can regulate recruited monocytes to differentiate into different macrophage subtypes, regulatory macrophages also differentiate from M1 and M2/wound healing activated macrophages, triggered by mechanisms that are poorly understood.
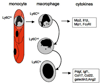
Figure 4. Ly6C, a marker of monocyte and macrophage heterogeneity
Schematic showing recruitment of Ly6Chi monocytes selectively into the kidney from capillaries, which differentiate into three populations of kidney macrophages, Ly6Chi, Ly6Cint and Ly6Clo. These kidney macrophage subpopulations generate discrete M1 biased (Ly6Chi) and M2 (Ly6Clo) biased cytokines in vivo. The Ly6Cint subpopulation comprises both macrophages derived from activation of resident macrophages and also macrophages in transition between with Ly6Chi and Ly6Clo subpopulations.
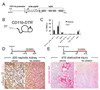
Figure 5. Studying macrophages in vivo by ablation using the CD11b-DTR mouse model
(A) The CD11b-DTR mouse harbors the CD11b-DTR transgene where the diphtheria toxin receptor is regulated by the CD11b promoter/enhancer. (B) By injecting minute amounts of DT into these mice only cells expressing the DTR are susceptible to its lethal effect. (C) Graph showing the effect of a single injection of DT on peripheral blood mononuclear cell populations (PBMCs). Note the selective ablation of CD11b+, Ly6G-, NK1.1 cells which are monocytes. Neutrophils are also not affected (not shown). (D) Schematic of late ablation of monocytes and Mϕs in the nephrotoxic nephritis (NTN) model of crescentic glomerulonephritis and Collagen-III stained lower power images of kidney d20 of NTN. Note the deposition of interstitial collagen is attenuated by Mϕ ablation as is tubular atrophy (E) Schematic of late ablation of monoctes and Mϕs in the chronic obstructive injury model and images of Sirius red stained sections of kidneys after 10d of injury. Note Mϕ ablation attenuated fibrosis in this model of chronic kidney disease also.
Figure 6. Schematics of proposed models of macrophage mediated fibrosis
(I) Arginase, TGFβ and IL13 have been shown in pathogen triggered liver fibrosis, lung fibrosis and skin diseases and kidney diseases (TGFβ only) to be significant Mϕ factors in fibrogenesis (II) Activated Mϕs differentiate into Type 2 (M2 or wound healing) Mϕs liberate cytokines that can drive pericyte or myofibroblast activation and consequent deposition of fibrillar collagens I and III, (III) A subpopulation of monocytes differentiates directly into a scar forming cell called fibrocyte (IV) Activated Mϕs injure endothelial cells which sequentially trigger pericyte migration and differentiation into myofibroblasts, or they injure epithelial cells which sequentially liberate factors that promote pericyte migration from capillaries and differentiation into myofibroblasts.
Figure 7. Mechanism by which Pentraxin-2/Serum Amyloid P inhibits macrophage directed fibrogenesis in the kidney
PTX-2 (red pentamers) opsonization of apoptotic cells, debris and oxidized matrix, triggers a conformational change that renders PTX-2 a high affinity ligand for activating immunoglobulin Fcγ receptors hFcγRIIA and III. Ligation of activating receptors on inflammatory kidney macrophages triggers differentiation of inflammatory Mϕs into regulatory Mϕs which generate IL10. This inhibits both Ly6Chi and Ly6Clo Mϕ activation and also directly inhibits collagen synthesis by myofibroblasts. In other organ systems PTX-2 has been reported to trigger differentiation of fibrocytes but these are not detected in kidney disease.
Similar articles
- Macrophages and kidney transplantation.
Chadban SJ, Wu H, Hughes J. Chadban SJ, et al. Semin Nephrol. 2010 May;30(3):278-89. doi: 10.1016/j.semnephrol.2010.03.009. Semin Nephrol. 2010. PMID: 20620672 Review. - The pattern recognition receptor, Mincle, is essential for maintaining the M1 macrophage phenotype in acute renal inflammation.
Lv LL, Tang PM, Li CJ, You YK, Li J, Huang XR, Ni J, Feng M, Liu BC, Lan HY. Lv LL, et al. Kidney Int. 2017 Mar;91(3):587-602. doi: 10.1016/j.kint.2016.10.020. Epub 2016 Dec 22. Kidney Int. 2017. PMID: 28017324 - Macrophages and renal fibrosis.
Vernon MA, Mylonas KJ, Hughes J. Vernon MA, et al. Semin Nephrol. 2010 May;30(3):302-17. doi: 10.1016/j.semnephrol.2010.03.004. Semin Nephrol. 2010. PMID: 20620674 Review. - Circulating CSF-1 promotes monocyte and macrophage phenotypes that enhance lupus nephritis.
Menke J, Rabacal WA, Byrne KT, Iwata Y, Schwartz MM, Stanley ER, Schwarting A, Kelley VR. Menke J, et al. J Am Soc Nephrol. 2009 Dec;20(12):2581-92. doi: 10.1681/ASN.2009050499. Epub 2009 Nov 19. J Am Soc Nephrol. 2009. PMID: 19926892 Free PMC article. - Targeting the recruitment of monocytes and macrophages in renal disease.
Vielhauer V, Kulkarni O, Reichel CA, Anders HJ. Vielhauer V, et al. Semin Nephrol. 2010 May;30(3):318-33. doi: 10.1016/j.semnephrol.2010.03.006. Semin Nephrol. 2010. PMID: 20620675 Review.
Cited by
- Complex Pathophysiology of Acute Kidney Injury (AKI) in Aging: Epigenetic Regulation, Matrix Remodeling, and the Healing Effects of H2S.
Gupta S, Mandal S, Banerjee K, Almarshood H, Pushpakumar SB, Sen U. Gupta S, et al. Biomolecules. 2024 Sep 17;14(9):1165. doi: 10.3390/biom14091165. Biomolecules. 2024. PMID: 39334931 Free PMC article. Review. - Clinical and preclinical studies of mesenchymal stem cells to alleviate peritoneal fibrosis.
Zheng L, Chen W, Yao K, Xie Y, Liao C, Zhou T. Zheng L, et al. Stem Cell Res Ther. 2024 Jul 30;15(1):237. doi: 10.1186/s13287-024-03849-3. Stem Cell Res Ther. 2024. PMID: 39080683 Free PMC article. Review. - Sterile inflammation of peritoneal membrane caused by peritoneal dialysis: focus on the communication between immune cells and peritoneal stroma.
Su H, Zou R, Su J, Chen X, Yang H, An N, Yang C, Tang J, Liu H, Yao C. Su H, et al. Front Immunol. 2024 May 8;15:1387292. doi: 10.3389/fimmu.2024.1387292. eCollection 2024. Front Immunol. 2024. PMID: 38779674 Free PMC article. Review. - Laboratory Analysis of the Renal Function Changes Under Long-Term Exposure to Extremely Low Ambient Temperatures: Case Report.
Teległów A, Skowron B, Romanovski V. Teległów A, et al. Ther Hypothermia Temp Manag. 2024 Mar;14(1):59-65. doi: 10.1089/ther.2023.0086. Epub 2024 Feb 23. Ther Hypothermia Temp Manag. 2024. PMID: 38394138 Free PMC article.
References
- van Furth R. Macrophage activity and clinical immunology. Origin and kinetics of mononuclear phagocytes. Ann N Y Acad Sci. 1976;278:161–175. - PubMed
- Magil AB, Wadsworth LD. Monocyte involvement in glomerular crescents: a histochemical and ultrastructural study. Lab Invest. 1982;47(2):160–166. - PubMed
- Germain MJ, Anderson RW, Keane WF. Renal disease in cryoglobulinemia type II: response to therapy. A case report and review of the literature. Am J Nephrol. 1982;2(4):221–226. - PubMed
- Ferrario F, Castiglione A, Colasanti G, et al. The detection of monocytes in human glomerulonephritis. Kidney Int. 1985;28(3):513–519. - PubMed
Publication types
MeSH terms
Grants and funding
- RC1 DK087389-01/DK/NIDDK NIH HHS/United States
- K08 DK073299/DK/NIDDK NIH HHS/United States
- K08 DK073299-04/DK/NIDDK NIH HHS/United States
- R01 DK084077-01/DK/NIDDK NIH HHS/United States
- DK84077/DK/NIDDK NIH HHS/United States
- RC1 DK087389/DK/NIDDK NIH HHS/United States
- R01 DK084077/DK/NIDDK NIH HHS/United States
- DK73299/DK/NIDDK NIH HHS/United States
- DK87389/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources