Primary tumor genotype is an important determinant in identification of lung cancer propagating cells - PubMed (original) (raw)
Primary tumor genotype is an important determinant in identification of lung cancer propagating cells
Stephen J Curtis et al. Cell Stem Cell. 2010.
Abstract
Successful cancer therapy requires the elimination or incapacitation of all tumor cells capable of regenerating a tumor. Therapeutic advances therefore necessitate the characterization of the cells that are able to propagate a tumor in vivo. We show an important link between tumor genotype and isolation of tumor-propagating cells (TPCs). Three mouse models of the most common form of human lung cancer each had TPCs with a unique cell-surface phenotype. The cell-surface marker Sca1 did not enrich for TPCs in tumors initiated with oncogenic Kras, and only Sca1-negative cells propagated EGFR mutant tumors. In contrast, Sca1-positive cells were enriched for tumor-propagating activity in Kras tumors with p53 deficiency. Primary tumors that differ in genotype at just one locus can therefore have tumor-propagating cell populations with distinct markers. Our studies show that the genotype of tumor samples must be considered in studies to identify, characterize, and target tumor-propagating cells.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Figures
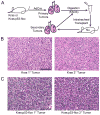
Figure 1
Orthotopic transplantation of Kras and Kras;p53-flox tumor cells recapitulates the primary tumor phenotype. (A) Cartoon of the transplantation scheme used to assay lung tumor-propagating cells (TPCs) through serial transplantation. (B) H&E staining of primary (left) and secondary (right) Kras lung adenocarcinomas showing similar pathological grade, nuclear features, and general tumor architecture. (C) H&E staining of primary (left) and secondary (right) Kras;p53-flox lung tumors showing similar histopathological characteristics of advanced adenocarcinoma, including pleomorphic nuclei and rare giant cells. All images, 200× magnification. Scale bar = 100μM.
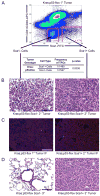
Figure 2
Sca1 + cells from Kras;p53-flox tumors are lung tumor-propagating cells. (A) Representative FACS analysis of Kras;p53-flox tumor cells used for transplantation (top). Limiting dilution transplantation of the sorted cells indicated that the Sca1 + population was significantly enriched for TPCs (table). (B) Sca1- cell transplants yielded smaller, more diffuse lesions (left), whereas Sca1 + cell transplants yielded secondary tumors that recapitulated the histopathology of primary Kras;p53-flox tumors (right, compare to Figure 1C). (C) Immunofluorescence (IF) staining with antisera raised against SP-C (red), CCSP (green), and counterstain DAPI (blue) showed that primary Kras;p53-flox lung adenocarcinomas (left) are mainly composed of SP-C+ cells, a pattern recapitulated in secondary tumors from Sca1 + cell transplants (right). (D) Serial transplantation of secondary tumor cell populations revealed a lack of tumor formation from Sca1 - cells (left), in contrast to tertiary tumor development from Sca1 + cells (right). All images, 200× magnification. Scale bar = 100μM. See also Figure S1 and Table S1 for additional data.
Figure 3
The Sca1 + and Sca1- populations from Kras lung tumors are equally capable of propagating tumors. (A) Representative FACS analysis of Kras tumors showing a pattern of Sca1 staining similar to Kras;p53-flox tumors (top, compare to Figure 2A). Limiting dilution transplantation indicated that Sca1+ and Sca1- Kras tumor cells are identical in tumor-propagating potential (table). (B) Secondary tumors from Sca1- (left) and Sca1+ (right) Kras tumor cell transplants displayed similar pathological features. (C) IF analysis of secondary tumors (as in Figure 2) revealed similar SP-C and CCSP marker status in secondary tumors from Sca1- cells (left) and Sca1+ cells (right). (D) Both Sca1- Kras tumor cells (left) and Sca1+ tumor cells (right) were capable of serial transplantation to form tertiary tumors. All images, 200× magnification. Scale bar = 100μM. See also Figure S2 and Table S2 for additional data.
Comment in
- Tumor oncogenotypes and lung cancer stem cell identity.
Sullivan JP, Minna JD. Sullivan JP, et al. Cell Stem Cell. 2010 Jul 2;7(1):2-4. doi: 10.1016/j.stem.2010.06.005. Cell Stem Cell. 2010. PMID: 20621039 Free PMC article.
Similar articles
- Identification and Targeting of Long-Term Tumor-Propagating Cells in Small Cell Lung Cancer.
Jahchan NS, Lim JS, Bola B, Morris K, Seitz G, Tran KQ, Xu L, Trapani F, Morrow CJ, Cristea S, Coles GL, Yang D, Vaka D, Kareta MS, George J, Mazur PK, Nguyen T, Anderson WC, Dylla SJ, Blackhall F, Peifer M, Dive C, Sage J. Jahchan NS, et al. Cell Rep. 2016 Jul 19;16(3):644-56. doi: 10.1016/j.celrep.2016.06.021. Epub 2016 Jun 30. Cell Rep. 2016. PMID: 27373157 Free PMC article. - A rare population of CD24(+)ITGB4(+)Notch(hi) cells drives tumor propagation in NSCLC and requires Notch3 for self-renewal.
Zheng Y, de la Cruz CC, Sayles LC, Alleyne-Chin C, Vaka D, Knaak TD, Bigos M, Xu Y, Hoang CD, Shrager JB, Fehling HJ, French D, Forrest W, Jiang Z, Carano RA, Barck KH, Jackson EL, Sweet-Cordero EA. Zheng Y, et al. Cancer Cell. 2013 Jul 8;24(1):59-74. doi: 10.1016/j.ccr.2013.05.021. Cancer Cell. 2013. PMID: 23845442 Free PMC article. - Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities.
Kerr EM, Gaude E, Turrell FK, Frezza C, Martins CP. Kerr EM, et al. Nature. 2016 Mar 3;531(7592):110-3. doi: 10.1038/nature16967. Epub 2016 Feb 24. Nature. 2016. PMID: 26909577 Free PMC article. - High-affinity peptide ligand LXY30 for targeting α3β1 integrin in non-small cell lung cancer.
Xiao W, Ma W, Wei S, Li Q, Liu R, Carney RP, Yang K, Lee J, Nyugen A, Yoneda KY, Lam KS, Li T. Xiao W, et al. J Hematol Oncol. 2019 Jun 10;12(1):56. doi: 10.1186/s13045-019-0740-7. J Hematol Oncol. 2019. PMID: 31182116 Free PMC article. - Targeting KRAS Mutant Non-Small-Cell Lung Cancer: Past, Present and Future.
Uras IZ, Moll HP, Casanova E. Uras IZ, et al. Int J Mol Sci. 2020 Jun 17;21(12):4325. doi: 10.3390/ijms21124325. Int J Mol Sci. 2020. PMID: 32560574 Free PMC article. Review.
Cited by
- βArrestin-1 and Mcl-1 modulate self-renewal growth of cancer stem-like side-population cells in non-small cell lung cancer.
Singh S, Bora-Singhal N, Kroeger J, Laklai H, Chellappan SP. Singh S, et al. PLoS One. 2013;8(2):e55982. doi: 10.1371/journal.pone.0055982. Epub 2013 Feb 13. PLoS One. 2013. PMID: 23418490 Free PMC article. - The side population in human lung cancer cell line NCI-H460 is enriched in stem-like cancer cells.
Shi Y, Fu X, Hua Y, Han Y, Lu Y, Wang J. Shi Y, et al. PLoS One. 2012;7(3):e33358. doi: 10.1371/journal.pone.0033358. Epub 2012 Mar 13. PLoS One. 2012. PMID: 22428030 Free PMC article. - Unravelling cancer stem cell potential.
Beck B, Blanpain C. Beck B, et al. Nat Rev Cancer. 2013 Oct;13(10):727-38. doi: 10.1038/nrc3597. Nat Rev Cancer. 2013. PMID: 24060864 Review. - Environment Impacts the Metabolic Dependencies of Ras-Driven Non-Small Cell Lung Cancer.
Davidson SM, Papagiannakopoulos T, Olenchock BA, Heyman JE, Keibler MA, Luengo A, Bauer MR, Jha AK, O'Brien JP, Pierce KA, Gui DY, Sullivan LB, Wasylenko TM, Subbaraj L, Chin CR, Stephanopolous G, Mott BT, Jacks T, Clish CB, Vander Heiden MG. Davidson SM, et al. Cell Metab. 2016 Mar 8;23(3):517-28. doi: 10.1016/j.cmet.2016.01.007. Epub 2016 Feb 4. Cell Metab. 2016. PMID: 26853747 Free PMC article. - p38α negatively regulates survival and malignant selection of transformed bronchioalveolar stem cells.
Voisset E, Oeztuerk-Winder F, Ruiz EJ, Ventura JJ. Voisset E, et al. PLoS One. 2013 Nov 12;8(11):e78911. doi: 10.1371/journal.pone.0078911. eCollection 2013. PLoS One. 2013. PMID: 24265727 Free PMC article.
References
- Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature medicine. 1997;3:730–737. - PubMed
- Cho RW, Wang X, Diehn M, Shedden K, Chen GY, Sherlock G, Gurney A, Lewicki J, Clarke MF. Isolation and molecular characterization of cancer stem cells in MMTV-Wnt-1 murine breast tumors. Stem Cells. 2008;26:364–371. - PubMed
Publication types
MeSH terms
Grants and funding
- K08 AG024004/AG/NIA NIH HHS/United States
- R01 CA122794/CA/NCI NIH HHS/United States
- R01 AG2400401/AG/NIA NIH HHS/United States
- R01 CA140594/CA/NCI NIH HHS/United States
- P50 CA090578/CA/NCI NIH HHS/United States
- R01 HL090136/HL/NHLBI NIH HHS/United States
- NIH/NCI 2P50CA090578/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous