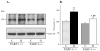Poly(ADP-ribose) polymerase-1 (PARP-1) gene deficiency alleviates diabetic kidney disease - PubMed (original) (raw)
Poly(ADP-ribose) polymerase-1 (PARP-1) gene deficiency alleviates diabetic kidney disease
Hanna Shevalye et al. Biochim Biophys Acta. 2010 Nov.
Abstract
Poly(ADP-ribose)polymerase (PARP) inhibitors prevent or alleviate diabetic nephropathy. This study evaluated the role for PARP-1 in diabetic kidney disease using the PARP-1-deficient mouse. PARP-1-/- and the wild-type (129S1/SvImJ) mice were made diabetic with streptozotocin, and were maintained for 12 weeks. Final blood glucose concentrations were increased ∼ 3.7-fold in both diabetic groups. PARP-1 protein expression (Western blot analysis) in the renal cortex was similar in non-diabetic and diabetic wild-type mice (100% and 107%) whereas all knockouts were PARP-1-negative. PARP-1 gene deficiency reduced urinary albumin (ELISA) and protein excretion prevented diabetes-induced kidney hypertrophy, and decreased mesangial expansion and collagen deposition (both assessed by histochemistry) as well as fibronectin expression. Renal podocyte loss (immunohistochemistry) and nitrotyrosine and transforming growth factor-β₁ accumulations (both by ELISA) were slightly lower in diabetic PARP-1-/- mice, but the differences with diabetic wild-type group did not achieve statistical significance. In conclusion, PARP-1-/- gene deficiency alleviates although does not completely prevent diabetic kidney disease.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
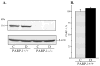
Fig. 1
A) Representative Western blot analysis of renal cortex poly(ADP-ribose) polymerase-1 and B) poly(ADP-ribose)polymerase-1 content (densitometry), in control and diabetic wild-type and poly(ADP-ribose) polymerase-1-deficient mice. C – control; D – diabetic, PARP-1 – poly(ADP-ribose) polymerase-1. Mean ± SEM, n = 8–10 per group.
Fig. 2
A) Representative Western blot analysis of renal cortex poly(ADP-ribosyl)ated proteins and B) poly(ADP-ribosyl)ated protein content (densitometry), in control and diabetic wild-type and poly(ADP-ribose)polymerase-1-deficient mice. C – control; D – diabetic, PARP-1 – poly(ADP-ribose) polymerase-1. Mean ± SEM, n = 9–12 per group. *, ** - p < 0.05 and < 0.01 vs corresponding non-diabetic groups.
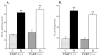
Fig. 3
A) Kidney weights and B) kidney weight-to-body weight ratios, in control and diabetic wild-type and poly(ADP-ribose)polymerase-1-deficient mice. C – control; D – diabetic, PARP-1 – poly(ADP-ribose) polymerase-1. Mean ± SEM, n = 10–15 per group. ** - p < 0.01 vs corresponding non-diabetic groups; ## - p < 0.01 vs diabetic wild-type mice.
Fig. 4
A) Representative microphotographs and B) color intensities of PAS-positive substance stainings in the renal cortex in control and diabetic wild-type and poly(ADP-ribose)polymerase-1-deficient mice. C – control; D – diabetic, PARP-1 – poly(ADP-ribose) polymerase-1. Magnification x 400. Mean ± SEM, n = 10 per group. ** - p < 0.01 vs corresponding non-diabetic groups; # - p < 0.05 vs diabetic wild-type mice.
Fig. 5
A) Representative Western blot analysis of renal cortex fibronectin and B) fibronectin content (densitometry), in control and diabetic wild-type and poly(ADP-ribose)polymerase-1-deficient mice. C – control; D – diabetic, PARP-1 – poly(ADP-ribose) polymerase-1. Mean ± SEM, n = 10–13 per group. *, ** - p < 0.05 and < 0.01 vs corresponding non-diabetic groups; ## - p < 0.01 vs diabetic wild-type mice.
Fig. 6
A) Representative microphotographs of glomerular podocyte immunostaining and B) podocyte counts, in control and diabetic wild-type and poly(ADP-ribose)polymerase-1-deficient mice. C – control; D – diabetic, PARP-1 – poly(ADP-ribose) polymerase-1. Magnification x 400. Mean ± SEM, n = 10 per group. ** - p < 0.01 vs corresponding non-diabetic groups.
Fig. 7
A) Transforming growth factor-β and B) nitrotyrosine concentrations, in the renal cortex in control and diabetic wild-type and poly(ADP-ribose)polymerase-1-deficient mice. C – control; D – diabetic, PARP-1 – poly(ADP-ribose) polymerase-1. Mean ± SEM, n = 9–14 per group. ** - p < 0.01 vs corresponding non-diabetic groups.
Fig. 8
A) Representative microphotographs and B) percentage of positively stained for collagen area in the renal cortex in control and diabetic wild-type and poly(ADP-ribose)polymerase-1-deficient mice. C – control; D – diabetic, PARP-1 – poly(ADP-ribose) polymerase-1. Magnification x 200. Mean ± SEM, n = 8–10 per group. ** - p < 0.01 vs corresponding non-diabetic groups; ## - p < 0.01 vs diabetic wild-type mice.
Similar articles
- Poly(Adenosine 5'-diphosphate-ribose) polymerase inhibition counteracts multiple manifestations of experimental type 1 diabetic nephropathy.
Drel VR, Xu W, Zhang J, Pavlov IA, Shevalye H, Slusher B, Obrosova IG. Drel VR, et al. Endocrinology. 2009 Dec;150(12):5273-83. doi: 10.1210/en.2009-0628. Epub 2009 Oct 23. Endocrinology. 2009. PMID: 19854869 Free PMC article. - Poly(ADP-ribose) polymerase (PARP) inhibition counteracts multiple manifestations of kidney disease in long-term streptozotocin-diabetic rat model.
Shevalye H, Stavniichuk R, Xu W, Zhang J, Lupachyk S, Maksimchyk Y, Drel VR, Floyd EZ, Slusher B, Obrosova IG. Shevalye H, et al. Biochem Pharmacol. 2010 Apr 1;79(7):1007-14. doi: 10.1016/j.bcp.2009.11.018. Epub 2009 Nov 27. Biochem Pharmacol. 2010. PMID: 19945439 Free PMC article. - Aldose reductase inhibition counteracts nitrosative stress and poly(ADP-ribose) polymerase activation in diabetic rat kidney and high-glucose-exposed human mesangial cells.
Drel VR, Pacher P, Stevens MJ, Obrosova IG. Drel VR, et al. Free Radic Biol Med. 2006 Apr 15;40(8):1454-65. doi: 10.1016/j.freeradbiomed.2005.12.034. Epub 2006 Jan 31. Free Radic Biol Med. 2006. PMID: 16631535 Free PMC article. - Poly(ADP-ribose)polymerase inhibition counteracts renal hypertrophy and multiple manifestations of peripheral neuropathy in diabetic Akita mice.
Drel VR, Pacher P, Stavniichuk R, Xu W, Zhang J, Kuchmerovska TM, Slusher B, Obrosova IG. Drel VR, et al. Int J Mol Med. 2011 Oct;28(4):629-35. doi: 10.3892/ijmm.2011.709. Epub 2011 May 23. Int J Mol Med. 2011. PMID: 21617845 Free PMC article. - Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model?
Shall S, de Murcia G. Shall S, et al. Mutat Res. 2000 Jun 30;460(1):1-15. doi: 10.1016/s0921-8777(00)00016-1. Mutat Res. 2000. PMID: 10856830 Review.
Cited by
- GYY4137, a Hydrogen Sulfide Donor Modulates miR194-Dependent Collagen Realignment in Diabetic Kidney.
John AMSP, Kundu S, Pushpakumar S, Fordham M, Weber G, Mukhopadhyay M, Sen U. John AMSP, et al. Sci Rep. 2017 Sep 7;7(1):10924. doi: 10.1038/s41598-017-11256-3. Sci Rep. 2017. PMID: 28883608 Free PMC article. - Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in wistar rats.
Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Braidy N, et al. PLoS One. 2011 Apr 26;6(4):e19194. doi: 10.1371/journal.pone.0019194. PLoS One. 2011. PMID: 21541336 Free PMC article. Retracted. - Prediabetic nephropathy as an early consequence of the high-calorie/high-fat diet: relation to oxidative stress.
Shevalye H, Lupachyk S, Watcho P, Stavniichuk R, Khazim K, Abboud HE, Obrosova IG. Shevalye H, et al. Endocrinology. 2012 Mar;153(3):1152-61. doi: 10.1210/en.2011-1997. Epub 2012 Jan 10. Endocrinology. 2012. PMID: 22234462 Free PMC article. - Parp1 protects against Aag-dependent alkylation-induced nephrotoxicity in a sex-dependent manner.
Calvo JA, Allocca M, Fake KR, Muthupalani S, Corrigan JJ, Bronson RT, Samson LD. Calvo JA, et al. Oncotarget. 2016 Jul 19;7(29):44950-44965. doi: 10.18632/oncotarget.10440. Oncotarget. 2016. PMID: 27391435 Free PMC article.
References
- Molitch ME, DeFronzo RA, Franz MJ, Keane WF, Mogensen CE, Parving HH, Steffes MW American Diabetes Association. Nephropathy in diabetes. Diabetes Care. 2004;27:S79–S83. - PubMed
- Nelson R, Knowler W, Pettitt D, Bennett P. National Institutes of Diabetes and Digestive and Kidney Diseases, editor. Diabetes in America, NIH Publication No.95–1468. Bethesda, MD: 1995. Kidney diseases in diabetes; pp. 349–385.
- Yuan H, Lanting L, Xu ZG, Li SL, Swiderski P, Putta S, Jonnalagadda M, Kato M, Natarajan R. Effects of cholesterol-tagged small interfering RNAs targeting 12/15-lipoxygenase on parameters of diabetic nephropathy in a mouse model of type 1 diabetes. Am J Physiol Renal Physiol. 2008;295:F605–F617. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1P30DK072476/DK/NIDDK NIH HHS/United States
- R01 DK074517/DK/NIDDK NIH HHS/United States
- R21 DK070720/DK/NIDDK NIH HHS/United States
- R01 DK077141/DK/NIDDK NIH HHS/United States
- P20 RR21945/RR/NCRR NIH HHS/United States
- DK077141/DK/NIDDK NIH HHS/United States
- DK070720/DK/NIDDK NIH HHS/United States
- P20 RR021945/RR/NCRR NIH HHS/United States
- DK074517/DK/NIDDK NIH HHS/United States
- P30 DK072476/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous