Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains - PubMed (original) (raw)
Rapid multiplex detection and differentiation of Listeria cells by use of fluorescent phage endolysin cell wall binding domains
Mathias Schmelcher et al. Appl Environ Microbiol. 2010 Sep.
Abstract
The genus Listeria comprises food-borne pathogens associated with severe infections and a high mortality rate. Endolysins from bacteriophages infecting Listeria are promising tools for both their detection and control. These proteins feature a modular organization, consisting of an N-terminal enzymatically active domain (EAD), which contributes lytic activity, and a C-terminal cell wall binding domain (CBD), which targets the lysin to its substrate. Sequence comparison among 12 different endolysins revealed high diversity among the enzyme's functional domains and allowed classification of their CBDs into two major groups and five subclasses. This diversity is reflected in various binding properties, as determined by cell wall binding assays using CBDs fused to fluorescent marker proteins. Although some proteins exhibited a broad binding range and recognize Listeria strains representing all serovars, others target specific serovars only. The CBDs also differed with respect to the number and distribution of ligands recognized on the cells, as well as their binding affinities. Surface plasmon resonance analysis revealed equilibrium affinities in the pico- to nanomolar ranges for all proteins except CBD006, which is due to an internal truncation. Rapid multiplexed detection and differentiation of Listeria strains in mixed bacterial cultures was possible by combining CBDs of different binding specificities with fluorescent markers of various colors. In addition, cells of different Listeria strains could be recovered from artificially contaminated milk or cheese by CBD-based magnetic separation by using broad-range CBDP40 and subsequently identified after incubation with two differently colored CBD fusion proteins of higher specificity.
Figures
FIG. 1.
Listeria bacteriophage endolysin domain structures and amino acid sequence relatedness. (A) Schematic alignment of nine different Listeria phage endolysins. The N-terminal EADs and C-terminal CBDs are marked according to the different homology groups and classes, respectively. Regions of sequence homology are indicated by black lines. Ply006 features a deletion of the N-terminal portion of its CBD, compared to Ply118. Catalytic activities are indicated. (B) Phylogenetic tree indicating the relationships between CBDs examined in the present study. For calculation of the tree, the respective amino acid sequences, including the putative linker regions, were used. The lengths of the branches correspond to evolutionary distances between the CBDs. The tree was used as basis for the classification of CBDs.
FIG. 2.
Pairwise amino acid sequence alignments of the border regions between EADs and CBDs of Listeria phage endolysins characterized in the present study. Identical and similar residues are marked in yellow and green, respectively. Putative linker regions between EADs and CBDs are indicated by black boxes. The linker of PlyPSA is known from the crystal structure (21).
FIG. 3.
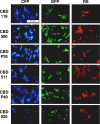
Listeria cells labeled with a set of 18 different fluorescent protein-CBD fusion proteins. Combinations of three different fluorescent reporters (CFP, cyan fluorescent protein; GFP, green fluorescent protein; RS, RedStar protein) and six different CBDs (one from each subclass) are shown. Listeria strains were selected according to the binding specificity of each CBD (see text). The samples were observed by epifluorescence microscopy using filter sets suitable for each fluorescent protein.
FIG. 4.
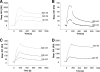
SPR analysis of the binding of CBD proteins to Listeria cell surfaces. The overlay plots show the binding kinetics of the indicated concentrations of HGFP-CBDP35 (A), HGFP-CBD006 (B), HGFP-CBD511 (C), and HGFP-CBDP40 (D) to L. monocytogenes cells immobilized on the sensor chip surface (see the text for explanation). The CBD association was monitored for 3 min; the subsequent dissociation was monitored for 12 min.
FIG. 5.
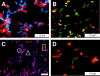
Differentiation of Listeria strains by multiplex fluorescent protein-CBD labeling (for details, see the text). (A) Cells of strains 1001 (sv. 1/2c) and 1042 (sv. 4b) incubated with HRS-CBD118 (red) and HCFP-CBD500 (blue), resulting in a serovar-specific cell wall decoration. (B) Strains 1066 (sv. 1/2b), 1020 (sv. 4a), and 1042 (sv. 4b) can be distinguished by using HRS-CBDP35 and HYFP-CBDPSA in a multiplex assay. Strain 1066 is labeled by CBD-P35 (red), strain 1042 is labeled by CBD-PSA (green), and strain WSLC 1020 is labeled by both CBDs (yellow). (C) Strains EGDe (sv. 1/2a), WSLC 1020 (sv. 4a), and WSLC 3010 (sv. 5) are distinguished by incubation with HRS-CBD511 and HBFP-CBD500. CBD511 strongly decorates WSLC 1020 and EGDe and only weakly binds to WSLC 3010, whereas CBD500 strongly binds to WSLC 1020 and 3010 and does not recognize EGDe cells. Therefore, strain EGDe appears red (square), WSLC 1020 is magenta (circle), and WSLC 3010 cells show up as purple (triangle). In panel D, detection and differentiation of Listeria strains CNL 103/2005 (1/2 a) and ScottA (4b) after recovery from contaminated milk and subsequent plating is shown, by a CBD binding assay. The green cells are strain ScottA, specifically tagged by HGFP-CBD500, while the red cells are CNL 103/2005 recognized by HRS-CBDP40. Note that deformation of the cells results from growth on drug-containing selective medium.
Similar articles
- Domain shuffling and module engineering of Listeria phage endolysins for enhanced lytic activity and binding affinity.
Schmelcher M, Tchang VS, Loessner MJ. Schmelcher M, et al. Microb Biotechnol. 2011 Sep;4(5):651-62. doi: 10.1111/j.1751-7915.2011.00263.x. Epub 2011 Apr 27. Microb Biotechnol. 2011. PMID: 21535426 Free PMC article. - Use of high-affinity cell wall-binding domains of bacteriophage endolysins for immobilization and separation of bacterial cells.
Kretzer JW, Lehmann R, Schmelcher M, Banz M, Kim KP, Korn C, Loessner MJ. Kretzer JW, et al. Appl Environ Microbiol. 2007 Mar;73(6):1992-2000. doi: 10.1128/AEM.02402-06. Epub 2007 Feb 2. Appl Environ Microbiol. 2007. PMID: 17277212 Free PMC article. - Use of bacteriophage cell wall-binding proteins for rapid diagnostics of Listeria.
Schmelcher M, Loessner MJ. Schmelcher M, et al. Methods Mol Biol. 2014;1157:141-56. doi: 10.1007/978-1-4939-0703-8_12. Methods Mol Biol. 2014. PMID: 24792555 - Biocontrol and Rapid Detection of Food-Borne Pathogens Using Bacteriophages and Endolysins.
Bai J, Kim YT, Ryu S, Lee JH. Bai J, et al. Front Microbiol. 2016 Apr 8;7:474. doi: 10.3389/fmicb.2016.00474. eCollection 2016. Front Microbiol. 2016. PMID: 27092128 Free PMC article. Review. - Bacteriophage endolysins: applications for food safety.
Schmelcher M, Loessner MJ. Schmelcher M, et al. Curr Opin Biotechnol. 2016 Feb;37:76-87. doi: 10.1016/j.copbio.2015.10.005. Epub 2015 Dec 18. Curr Opin Biotechnol. 2016. PMID: 26707470 Review.
Cited by
- The Application of Bacteriophage Diagnostics for Bacterial Pathogens in the Agricultural Supply Chain: From Farm-to-Fork.
Jones HJ, Shield CG, Swift BMC. Jones HJ, et al. Phage (New Rochelle). 2020 Dec 1;1(4):176-188. doi: 10.1089/phage.2020.0042. Epub 2020 Dec 16. Phage (New Rochelle). 2020. PMID: 36147287 Free PMC article. Review. - Characterization of antibacterial activity of a N-acetylmuramoyl-L-alanine amidase produced by Latilactobacillus sakei isolated from salami.
Lopez-Arvizu A, Rocha-Mendoza D, Ponce-Alquicira E, García-Cano I. Lopez-Arvizu A, et al. World J Microbiol Biotechnol. 2021 Mar 19;37(4):65. doi: 10.1007/s11274-021-03033-2. World J Microbiol Biotechnol. 2021. PMID: 33740141 - Enzybiotics: Enzyme-Based Antibacterials as Therapeutics.
Dams D, Briers Y. Dams D, et al. Adv Exp Med Biol. 2019;1148:233-253. doi: 10.1007/978-981-13-7709-9_11. Adv Exp Med Biol. 2019. PMID: 31482502 Review. - Bacteriophages in Natural and Artificial Environments.
Batinovic S, Wassef F, Knowler SA, Rice DTF, Stanton CR, Rose J, Tucci J, Nittami T, Vinh A, Drummond GR, Sobey CG, Chan HT, Seviour RJ, Petrovski S, Franks AE. Batinovic S, et al. Pathogens. 2019 Jul 12;8(3):100. doi: 10.3390/pathogens8030100. Pathogens. 2019. PMID: 31336985 Free PMC article. Review. - A comprehensive review of the applications of bacteriophage-derived endolysins for foodborne bacterial pathogens and food safety: recent advances, challenges, and future perspective.
Khan FM, Chen JH, Zhang R, Liu B. Khan FM, et al. Front Microbiol. 2023 Oct 6;14:1259210. doi: 10.3389/fmicb.2023.1259210. eCollection 2023. Front Microbiol. 2023. PMID: 37869651 Free PMC article. Review.
References
- Bateman, A., and M. Bycroft. 2000. The structure of a LysM domain from Escherichia coli membrane-bound lytic murein transglycosylase D (MltD). J. Mol. Biol. 299:1113-1119. - PubMed
- Bille, J., D. S. Blanc, H. Schmid, K. Boubaker, A. Baumgartner, H. H. Siegrist, M. L. Tritten, R. Lienhard, D. Berner, R. Anderau, M. Treboux, J. M. Ducommun, R. Malinverni, D. Genne, P. H. Erard, and U. Waespi. 2006. Outbreak of human listeriosis associated with Tomme cheese in northwest Switzerland, 2005. Euro Surveill. 11:91-93. - PubMed
- Dorscht, J., J. Klumpp, R. Bielmann, M. Schmelcher, Y. Born, M. Zimmer, R. Calendar, and M. J. Loessner. 2009. Comparative genome analysis of Listeria bacteriophages reveals extensive mosaicism, programmed translational frameshifting, and a novel prophage insertion site. J. Bacteriol. 191:7206-7215. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources