Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats - PubMed (original) (raw)
. 2010 Oct;59(10):2653-61.
doi: 10.2337/db09-1564. Epub 2010 Jul 9.
Affiliations
- PMID: 20622169
- PMCID: PMC3279561
- DOI: 10.2337/db09-1564
Chronic administration of the glucagon-like peptide-1 analog, liraglutide, delays the onset of diabetes and lowers triglycerides in UCD-T2DM rats
Bethany P Cummings et al. Diabetes. 2010 Oct.
Abstract
Objective: The efficacy of liraglutide, a human glucagon-like peptide-1 (GLP-1) analog, to prevent or delay diabetes in UCD-T2DM rats, a model of polygenic obese type 2 diabetes, was investigated.
Research design and methods: At 2 months of age, male rats were divided into three groups: control, food-restricted, and liraglutide. Animals received liraglutide (0.2 mg/kg s.c.) or vehicle injections twice daily. Restricted rats were food restricted to equalize body weights to liraglutide-treated rats. Half of the animals were followed until diabetes onset, whereas the other half of the animals were killed at 6.5 months of age for tissue collection.
Results: Before diabetes onset energy intake, body weight, adiposity, and liver triglyceride content were higher in control animals compared with restricted and liraglutide-treated rats. Energy-restricted animals had lower food intake than liraglutide-treated animals to maintain the same body weights, suggesting that liraglutide increases energy expenditure. Liraglutide treatment delayed diabetes onset by 4.1 ± 0.8 months compared with control (P < 0.0001) and by 1.3 ± 0.8 months compared with restricted animals (P < 0.05). Up to 6 months of age, energy restriction and liraglutide treatment lowered fasting plasma glucose and A1C concentrations compared with control animals. In contrast, liraglutide-treated animals exhibited lower fasting plasma insulin, glucagon, and triglycerides compared with both control and restricted animals. Furthermore, energy-restricted and liraglutide-treated animals exhibited more normal islet morphology.
Conclusions: Liraglutide treatment delays the development of diabetes in UCD-T2DM rats by reducing energy intake and body weight, and by improving insulin sensitivity, improving lipid profiles, and maintaining islet morphology.
Figures
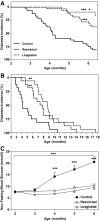
FIG. 1.
Kaplan-Meier analysis of diabetes incidence in control, restricted, and liraglutide-treated animals up to 6 months (n = 32 per group) (A) and 12 months (n = 16 per group) (B) of age. ***P < 0.0001, **P < 0.01 compared with control; *P < 0.05 compared with the food-restricted group by log-rank test. Nonfasting blood glucose in control, restricted, and liraglutide-treated animals (C). ***P < 0.0001 by two-factor (time and treatment) repeated-measures ANOVA, ***P < 0.001 compared with restricted and liraglutide-treated animals by Bonferroni post-test. Control: n = 27; restricted: n = 31; liraglutide-treated animals: n = 32.
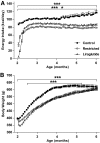
FIG. 2.
Energy intake (A) and body weight (B) in control, restricted, and liraglutide-treated animals. ***P < 0.0001 by two-factor (time and treatment) repeated-measures ANOVA; ***P < 0.001 compared with restricted and liraglutide-treated animals; *P < 0.05 liraglutide-treated animals versus restricted by Bonferroni post-test. Control: n = 27; restricted: n = 31; liraglutide-treated animals: n = 32.
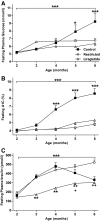
FIG. 3.
Fasting circulating glucose (A), A1C (B), and insulin (C) in control, restricted, and liraglutide-treated animals. ***P < 0.0001 by two-factor (time and treatment) repeated-measures ANOVA; ***P < 0.001, *P < 0.05 compared with restricted and liraglutide-treated animals; **P < 0.001 compared with restricted and control; ++P < 0.001 compared with restricted; +P < 0.05 compared with control by Bonferroni post-test. Control: n = 27; restricted: n = 31; liraglutide-treated animals: n = 32.
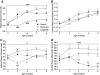
FIG. 4.
Fasting plasma TG (A), cholesterol (B), glucagon (C), and adiponectin (D) in control, food-restricted, and liraglutide-treated animals. ***P < 0.0001, *P < 0.05 by two-factor (time and treatment) repeated-measures ANOVA; ***P < 0.001 compared with food-restricted and control; **P < 0.001 compared with food-restricted; *P < 0.05 compared with control; +P < 0.05 compared with food-restricted; ++P < 0.05 compared with food-restricted and liraglutide-treated animals by Bonferroni post-test. Control: n = 27; restricted: n = 31; liraglutide-treated animals: n = 32.
FIG. 5.
Representative pancreas sections from control, food-restricted, and liraglutide-treated animals at 6 months of age. Hematoxylin and eosin staining for control (A), restricted (D), and liraglutide-treated animals (G) is shown. Anti-insulin immunostaining for control (B), restricted (E), and liraglutide-treated animals (H) is shown. Anti-glucagon immunostaining for control (C), restricted (F), and liraglutide-treated animals (I) is shown. (A high-quality digital representation of this figure is available in the online issue.)
Similar articles
- Growth hormone treatment does not augment the anti-diabetic effects of liraglutide in UCD-T2DM rats.
Swarbrick MM, Cox CL, Graham JL, Knudsen LB, Stanhope K, Raun K, Havel PJ. Swarbrick MM, et al. Endocrinol Diabetes Metab. 2023 Jan;6(1):e392. doi: 10.1002/edm2.392. Epub 2022 Dec 8. Endocrinol Diabetes Metab. 2023. PMID: 36480511 Free PMC article. - The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes.
Harder H, Nielsen L, Tu DT, Astrup A. Harder H, et al. Diabetes Care. 2004 Aug;27(8):1915-21. doi: 10.2337/diacare.27.8.1915. Diabetes Care. 2004. PMID: 15277417 Clinical Trial. - Liraglutide, a long-acting glucagon-like peptide-1 analog, reduces body weight and food intake in obese candy-fed rats, whereas a dipeptidyl peptidase-IV inhibitor, vildagliptin, does not.
Raun K, von Voss P, Gotfredsen CF, Golozoubova V, Rolin B, Knudsen LB. Raun K, et al. Diabetes. 2007 Jan;56(1):8-15. doi: 10.2337/db06-0565. Diabetes. 2007. PMID: 17192459 - Liraglutide for the treatment of type 2 diabetes: a clinical update.
Peters KR. Peters KR. Am J Ther. 2013 Mar-Apr;20(2):178-88. doi: 10.1097/MJT.0b013e3182204c16. Am J Ther. 2013. PMID: 21734532 Review. - Liraglutide: the therapeutic promise from animal models.
Knudsen LB. Knudsen LB. Int J Clin Pract Suppl. 2010 Oct;(167):4-11. doi: 10.1111/j.1742-1241.2010.02499.x. Int J Clin Pract Suppl. 2010. PMID: 20887299 Review.
Cited by
- Microneedle patch with pure drug tips for delivery of liraglutide: pharmacokinetics in rats and minipigs.
Lin H, Liu J, Hou Y, Yu Z, Hong J, Yu J, Chen Y, Hu J, Xia D. Lin H, et al. Drug Deliv Transl Res. 2024 Apr 15. doi: 10.1007/s13346-024-01582-1. Online ahead of print. Drug Deliv Transl Res. 2024. PMID: 38619705 - The Role of Obesity in Type 2 Diabetes Mellitus-An Overview.
Chandrasekaran P, Weiskirchen R. Chandrasekaran P, et al. Int J Mol Sci. 2024 Feb 4;25(3):1882. doi: 10.3390/ijms25031882. Int J Mol Sci. 2024. PMID: 38339160 Free PMC article. Review. - Contribution of animal models to diabetes research: Its history, significance, and translation to humans.
Yagihashi S. Yagihashi S. J Diabetes Investig. 2023 Sep;14(9):1015-1037. doi: 10.1111/jdi.14034. Epub 2023 Jul 3. J Diabetes Investig. 2023. PMID: 37401013 Free PMC article. Review. - Growth hormone treatment does not augment the anti-diabetic effects of liraglutide in UCD-T2DM rats.
Swarbrick MM, Cox CL, Graham JL, Knudsen LB, Stanhope K, Raun K, Havel PJ. Swarbrick MM, et al. Endocrinol Diabetes Metab. 2023 Jan;6(1):e392. doi: 10.1002/edm2.392. Epub 2022 Dec 8. Endocrinol Diabetes Metab. 2023. PMID: 36480511 Free PMC article. - Zucker Diabetic-Sprague Dawley (ZDSD) rat: Type 2 diabetes translational research model.
Wang AN, Carlos J, Fraser GM, McGuire JJ. Wang AN, et al. Exp Physiol. 2022 Apr;107(4):265-282. doi: 10.1113/EP089947. Epub 2022 Mar 8. Exp Physiol. 2022. PMID: 35178802 Free PMC article. Review.
References
- Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology 2007;132:2131–2157 - PubMed
- Herman GA, Stein PP, Thornberry NA, Wagner JA. Dipeptidyl peptidase-4 inhibitors for the treatment of type 2 diabetes: focus on sitagliptin. Clin Pharmacol Ther 2007;81:761–767 - PubMed
- Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet 1987;2:1300–1304 - PubMed
- Kim Chung le T, Hosaka T, Yoshida M, Harada N, Sakaue H, Sakai T, Nakaya Y. Exendin-4, a GLP-1 receptor agonist, directly induces adiponectin expression through protein kinase A pathway and prevents inflammatory adipokine expression. Biochem Biophys Res Commun 2009;390:613–618 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01-HL09333/HL/NHLBI NIH HHS/United States
- AT0-03545/AT/NCCIH NIH HHS/United States
- R21 AT002599/AT/NCCIH NIH HHS/United States
- F32 HL009333/HL/NHLBI NIH HHS/United States
- AT0-02993/AT/NCCIH NIH HHS/United States
- R21 AT002993/AT/NCCIH NIH HHS/United States
- P30 DK-17047/DK/NIDDK NIH HHS/United States
- AT0-02599/AT/NCCIH NIH HHS/United States
- P30 DK017047/DK/NIDDK NIH HHS/United States
- R01 HL075675/HL/NHLBI NIH HHS/United States
- R01-HL075675/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical