Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity - PubMed (original) (raw)
. 2010 Aug 2;207(8):1599-608.
doi: 10.1084/jem.20091663. Epub 2010 Jul 12.
Roopa Bhat, Daniel S Straus, Raymond A Sobel, Robert Axtell, Amanda Johnson, Kim Nguyen, Lata Mukundan, Marina Moshkova, Jason C Dugas, Ajay Chawla, Lawrence Steinman
Affiliations
- PMID: 20624891
- PMCID: PMC2916127
- DOI: 10.1084/jem.20091663
Peroxisome proliferator-activated receptor delta limits the expansion of pathogenic Th cells during central nervous system autoimmunity
Shannon E Dunn et al. J Exp Med. 2010.
Abstract
Peroxisome proliferator-activated receptors (PPARs; PPAR-alpha, PPAR-delta, and PPAR-gamma) comprise a family of nuclear receptors that sense fatty acid levels and translate this information into altered gene transcription. Previously, it was reported that treatment of mice with a synthetic ligand activator of PPAR-delta, GW0742, ameliorates experimental autoimmune encephalomyelitis (EAE), indicating a possible role for this nuclear receptor in the control of central nervous system (CNS) autoimmune inflammation. We show that mice deficient in PPAR-delta (PPAR-delta(-/-)) develop a severe inflammatory response during EAE characterized by a striking accumulation of IFN-gamma(+)IL-17A(-) and IFN-gamma(+)IL-17A(+) CD4(+) cells in the spinal cord. The preferential expansion of these T helper subsets in the CNS of PPAR-delta(-/-) mice occurred as a result of a constellation of immune system aberrations that included higher CD4(+) cell proliferation, cytokine production, and T-bet expression and enhanced expression of IL-12 family cytokines by myeloid cells. We also show that the effect of PPAR-delta in inhibiting the production of IFN-gamma and IL-12 family cytokines is ligand dependent and is observed in both mouse and human immune cells. Collectively, these findings suggest that PPAR-delta serves as an important molecular brake for the control of autoimmune inflammation.
Figures
Figure 1.
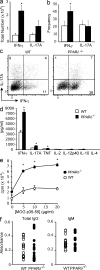
PPAR-δ−/− mice develop hyperacute EAE. EAE was induced in WT or PPAR-δ−/− female (a) or male (b–e) mice (n = 8–10/group). (a and b) Mean ± SEM clinical scores of mice at various times after immunization. Results are representative of two to three experiments. (c–e) Paraffin sections of spinal cord and brain were prepared from PPAR-δ−/− and WT mice at the time of first remission and were stained with H&E and luxol fast blue. d and e show representative sections (bar, 50 µM); c shows the mean + SEM number of inflammatory foci; g shows the regional distribution of parenchymal lesions. (f) Mononuclear cells were isolated from spinal cords of WT or PPAR-δ−/− mice (n = 3/group) at 4 d after the onset of clinical signs, and then they were pooled and counted. Cells were stained with antibodies specific for cell surface markers as indicated and the percentage of each subset in the live CD45+ gate is shown. Values are mean + SEM numbers from four experiments. *, significantly different from WT.
Figure 2.
PPAR-δ−/− mice exhibit higher Th cytokine production during EAE. (a and b) Mononuclear cells were isolated from spinal cords of WT or PPAR-δ−/− mice at 4 d after the onset of clinical signs, and then they were pooled (n = 3/group) and processed for intracellular cytokine staining. The number (a) and frequency (b) of CD4+ mononuclear cells that had intracellular accumulation of IFN-γ (IFN-γ+IL-17A− and IFN-γ+IL-17A+) or IL-17A (IL-17A+ IFN-γ+ and IL-17A+ IFN-γ−) are shown. (c) Representative FACs plot of IFN-γ and IL-17A staining in CD4+ gate. (d and e) Splenocytes from male age-matched WT or PPAR-δ−/− mice were harvested at 8 d after immunization and were stimulated ex vivo with MOG p35-55. Cytokines (d) were measured in culture supernatants using ELISA. Proliferation (48 h; e) was measured by [3H]-thymidine incorporation assay. Values are means + SEM of triplicate culture wells. Data in a–e are representative of three experiments. (f) Serum was collected from WT and PPAR-δ−/− mice during EAE. Titers of MOG p35-55–specific IgM and total IgG were determined by ELISA. Values are absorbance measurements of individual mice from two experiments that contained n = 5 and n = 11 mice/group. *, significantly different from WT.
Figure 3.
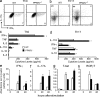
PPAR-δ limits cytokine production by CD4+ cells. (a–c) Naive CD4+ cells were isolated from the spleens of WT or PPAR-δ−/− mice and stimulated with anti-CD3 and anti-CD28 under Th0-skewing (a, c, e, and g) or Th17-skewing (b, d, f, and h), conditions. After 4 d of culture, CD4+ cells were processed for intracellular cytokine staining. (a and b) Representative FACs plots of events in the CD4+ gate. (c and d) Cytokine levels in culture supernatants after 48 h of culture under Th0-skewing (c) or Th17-skewing (d) conditions. (e–h) mRNA levels of IFN-γ and T-bet (Th0 conditions) or IL-17A and RORγt (Th17 conditions; relative to β-actin mRNAs) were determined using real-time PCR. *, significantly different from WT. Values are means + SEM of triplicate cultures (c and d) or triplicate reactions (e–h). Data are representative of two to five experiments.
Figure 4.
CD4+ T cells from PPAR-δ−/− mice are hyperresponsive. Naive CD4+ (a) or CD19+ (b) cells were isolated from the spleens of WT or PPAR-δ−/− mice and were stimulated as indicated. Proliferation of these cells was measured using a [3H]-thymidine incorporation assay. Values are means + SEM of triplicate cultures. (c and d) Splenocytes were harvested from nonimmunized WT or PPAR-δ−/− mice. The frequency of CD4+ cells that displayed a memory (CD44high and CD62Llow; c) or activated (CD69+ or CD25+; d) phenotype in each group was determined. In d, values represent mean + SEM frequencies of n = 3–4 mice/group. Data in a–d are representative of two experiments. *, significantly different from WT.
Figure 5.
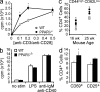
PPAR-δ negatively regulates expression of IL-12 family cytokines. Splenic CD11b+ cells (a and c) or peritoneal macrophages (b) were isolated from adult age-matched WT and PPAR-δ−/− mice and were cultured with 10 ng/ml LPS. (a and b) Supernatants were collected at 24 h for cytokine measurement by ELISA. (c) Cells were harvested at 0, 1, 3, or 5 h after stimulation for collection of RNA for real-time PCR measurement of p40, p35, or p19 mRNA expression (relative to β-actin). Values represent mean + SEM of triplicate cultures (a and b) or reactions (c). *, significantly different from WT. Results are representative of three experiments.
Figure 6.
GW0742 suppresses IFN-γ and IL-12 family cytokines in a PPAR-δ–dependent manner. CD4+ cells (a–e) or peritoneal macrophages (f) were stimulated in the presence of 100 nM GW0742 or vehicle of equal volume. Cells were cultured in complete RPMI (a) or X-vivo 20 serum-free media (b–f). CD4+ cells were stimulated with anti-CD3 under Th0-skewing (a–d) or Th17-skewing (e) conditions, whereas peritoneal macrophages were stimulated with LPS. Cytokine production was measured after 24 or 48 h of culture by ELISA and intracellular staining was conducted after 4 d of culture as in Fig. 3. Values represent means + SEM of triplicate cultures. *, significantly different from WT. †, significantly different from vehicle counterpart. Results are representative of three experiments.
Similar articles
- Peroxisome proliferator-activated receptor delta agonists inhibit T helper type 1 (Th1) and Th17 responses in experimental allergic encephalomyelitis.
Kanakasabai S, Chearwae W, Walline CC, Iams W, Adams SM, Bright JJ. Kanakasabai S, et al. Immunology. 2010 Aug;130(4):572-88. doi: 10.1111/j.1365-2567.2010.03261.x. Epub 2010 Apr 6. Immunology. 2010. PMID: 20406305 Free PMC article. - Peroxisome Proliferator-Activated Receptor-δ Acts within Peripheral Myeloid Cells to Limit Th Cell Priming during Experimental Autoimmune Encephalomyelitis.
Drohomyrecky PC, Doroshenko ER, Akkermann R, Moshkova M, Yi TJ, Zhao FL, Ahn JJ, McGaha TL, Pahan K, Dunn SE. Drohomyrecky PC, et al. J Immunol. 2019 Nov 15;203(10):2588-2601. doi: 10.4049/jimmunol.1801200. Epub 2019 Oct 2. J Immunol. 2019. PMID: 31578267 - RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation.
Codarri L, Gyülvészi G, Tosevski V, Hesske L, Fontana A, Magnenat L, Suter T, Becher B. Codarri L, et al. Nat Immunol. 2011 Jun;12(6):560-7. doi: 10.1038/ni.2027. Epub 2011 Apr 24. Nat Immunol. 2011. PMID: 21516112 - Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.
Rostami A, Ciric B. Rostami A, et al. J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review. - Nuclear receptors and autoimmune disease: the potential of PPAR agonists to treat multiple sclerosis.
Racke MK, Gocke AR, Muir M, Diab A, Drew PD, Lovett-Racke AE. Racke MK, et al. J Nutr. 2006 Mar;136(3):700-3. doi: 10.1093/jn/136.3.700. J Nutr. 2006. PMID: 16484546 Free PMC article. Review.
Cited by
- A role for Peroxisome Proliferator-Activated Receptor Beta in T cell development.
Mothe-Satney I, Murdaca J, Sibille B, Rousseau AS, Squillace R, Le Menn G, Rekima A, Larbret F, Pelé J, Verhasselt V, Grimaldi PA, Neels JG. Mothe-Satney I, et al. Sci Rep. 2016 Sep 29;6:34317. doi: 10.1038/srep34317. Sci Rep. 2016. PMID: 27680392 Free PMC article. - Dissecting the role of peroxisome proliferator-activated receptor-β/δ (PPARβ/δ) in colon, breast, and lung carcinogenesis.
Peters JM, Foreman JE, Gonzalez FJ. Peters JM, et al. Cancer Metastasis Rev. 2011 Dec;30(3-4):619-40. doi: 10.1007/s10555-011-9320-1. Cancer Metastasis Rev. 2011. PMID: 22037942 Free PMC article. Review. - Sex-specific regulation of immune responses by PPARs.
Park HJ, Choi JM. Park HJ, et al. Exp Mol Med. 2017 Aug 4;49(8):e364. doi: 10.1038/emm.2017.102. Exp Mol Med. 2017. PMID: 28775365 Free PMC article. Review. - Suppression of Experimental Autoimmune Encephalomyelitis in Mice by β-Hydroxy β-Methylbutyrate, a Body-Building Supplement in Humans.
Sheinin M, Mondal S, Roy A, Gorai S, Rangasamy SB, Poddar J, Pahan K. Sheinin M, et al. J Immunol. 2023 Jul 15;211(2):187-198. doi: 10.4049/jimmunol.2200267. J Immunol. 2023. PMID: 37314416 Free PMC article. - Recent Insights on the Role of PPAR-β/δ in Neuroinflammation and Neurodegeneration, and Its Potential Target for Therapy.
Strosznajder AK, Wójtowicz S, Jeżyna MJ, Sun GY, Strosznajder JB. Strosznajder AK, et al. Neuromolecular Med. 2021 Mar;23(1):86-98. doi: 10.1007/s12017-020-08629-9. Epub 2020 Nov 18. Neuromolecular Med. 2021. PMID: 33210212 Free PMC article. Review.
References
- Axtell R.C., Xu L., Barnum S.R., Raman C. 2006. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J. Immunol. 177:8542–8549 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK062386/DK/NIDDK NIH HHS/United States
- T32 AI007290/AI/NIAID NIH HHS/United States
- HL076746/HL/NHLBI NIH HHS/United States
- R01 HL076746/HL/NHLBI NIH HHS/United States
- K08 DK062386/DK/NIDDK NIH HHS/United States
- 835/MSS_/Multiple Sclerosis Society/United Kingdom
- R01 EY010257/EY/NEI NIH HHS/United States
- DK081405/DK/NIDDK NIH HHS/United States
- 5 R01 EY10257-12/EY/NEI NIH HHS/United States
- R01 DK081405/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials