Estimating the number of paediatric fevers associated with malaria infection presenting to Africa's public health sector in 2007 - PubMed (original) (raw)
Estimating the number of paediatric fevers associated with malaria infection presenting to Africa's public health sector in 2007
Peter W Gething et al. PLoS Med. 2010.
Abstract
Background: As international efforts to increase the coverage of artemisinin-based combination therapy in public health sectors gather pace, concerns have been raised regarding their continued indiscriminate presumptive use for treating all childhood fevers. The availability of rapid-diagnostic tests to support practical and reliable parasitological diagnosis provides an opportunity to improve the rational treatment of febrile children across Africa. However, the cost effectiveness of diagnosis-based treatment polices will depend on the presumed numbers of fevers harbouring infection. Here we compute the number of fevers likely to present to public health facilities in Africa and the estimated number of these fevers likely to be infected with Plasmodium falciparum malaria parasites.
Methods and findings: We assembled first administrative-unit level data on paediatric fever prevalence, treatment-seeking rates, and child populations. These data were combined in a geographical information system model that also incorporated an adjustment procedure for urban versus rural areas to produce spatially distributed estimates of fever burden amongst African children and the subset likely to present to public sector clinics. A second data assembly was used to estimate plausible ranges for the proportion of paediatric fevers seen at clinics positive for P. falciparum in different endemicity settings. We estimated that, of the 656 million fevers in African 0-4 y olds in 2007, 182 million (28%) were likely to have sought treatment in a public sector clinic of which 78 million (43%) were likely to have been infected with P. falciparum (range 60-103 million).
Conclusions: Spatial estimates of childhood fevers and care-seeking rates can be combined with a relational risk model of infection prevalence in the community to estimate the degree of parasitemia in those fevers reaching public health facilities. This quantification provides an important baseline comparison of malarial and nonmalarial fevers in different endemicity settings that can contribute to ongoing scientific and policy debates about optimum clinical and financial strategies for the introduction of new diagnostics. These models are made publicly available with the publication of this paper.
Conflict of interest statement
RWS chaired Novartis's National Malaria Control Programme Best Practice Workshops for several years in Africa for which he was paid an honorium. The need for defining drug commodity requirements by countries stems from these workshops. RWS thanks Novartis and the national programme participants of these workshops. Novartis has not, however, influenced the design of the data assembly, analysis, or interpretation of the results presented in this paper.
Figures
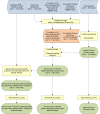
Figure 1. Schematic overview of mapping procedures and methods.
Blue rods describe input data; yellow boxes denote operations in a geographical information system; orange rods denote adjusted data; green rods indicate output data, with dashed lines denoting intermediate output and solid lines final outputs. U5, children aged under 5 y old.
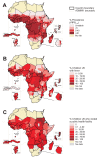
Figure 2. Transmission intensity, fevers, and care seeking for fever across Africa.
(A) Predicted transmission intensity across Africa. Transmission is classified into areas of risk free, unstable, and stable transmission on the basis of country-reported case data and the limiting effects on transmission of aridity and low temperatures . The latter class is further divided into low, medium, and high transmission settings from a model-based geostatistical prediction of P. falciparum prevalence in the epidemiologically informative 2-y up to 10-y age range, PfPR2–10. (B) 14-d period prevalence of reported fevers among children aged 0–4 y derived from national sample survey data (yellow, no risk; grey, no data). (C) Proportion of paediatric fevers using a public health facility at some stage of the illness to treat the fever (yellow, no risk; grey, no data). Footnote: The reference ADMIN1 digital boundaries for Africa were obtained through a combination of data from the United Nations Geographic Information Working Group, Second Administrative Level Boundary project (UNGIWG-SALB [56]) and the Food & Agriculture Organization - Global Administrative Units Layers (FAO-GAUL [57]). These boundary units matched reported information on fever prevalence for 31 of 42 national survey reports assembled. For Angola, Burundi, Central African Republic, Chad, Congo, Gabon, Guinea Bissau, Mauritania, and Nigeria nonstandard ADMIN1 units were reported by the national sample surveys and these were digitized using ArcGIS 9.3 (ESRI, Inc.) to replace existing ADMIN1 boundaries and thus create a single fever spatial reporting surface, similar to recent approaches to assemble mosquito net use from national survey data .
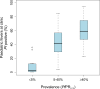
Figure 3. Risks of febrile children being infected when presenting to clinics within three epidemiological strata of unstable/≤5% PfPR2–10, >5% to <40% PfPR2–10, and ≥40% PfPR2–10.
The box indicates the IQR (25% and 75%); the thick line within the box represents the median; the whiskers represent the 2.5% and 97.5% centiles; and outliers are plotted as circles outside this range.
Similar articles
- The contribution of non-malarial febrile illness co-infections to Plasmodium falciparum case counts in health facilities in sub-Saharan Africa.
Dalrymple U, Cameron E, Arambepola R, Battle KE, Chestnutt EG, Keddie SH, Twohig KA, Pfeffer DA, Gibson HS, Weiss DJ, Bhatt S, Gething PW. Dalrymple U, et al. Malar J. 2019 Jun 11;18(1):195. doi: 10.1186/s12936-019-2830-y. Malar J. 2019. PMID: 31186004 Free PMC article. - Diagnostic testing of pediatric fevers: meta-analysis of 13 national surveys assessing influences of malaria endemicity and source of care on test uptake for febrile children under five years.
Johansson EW, Gething PW, Hildenwall H, Mappin B, Petzold M, Peterson SS, Selling KE. Johansson EW, et al. PLoS One. 2014 Apr 18;9(4):e95483. doi: 10.1371/journal.pone.0095483. eCollection 2014. PLoS One. 2014. PMID: 24748201 Free PMC article. - Diagnosis and Treatment of the Febrile Child.
Herlihy JM, D’Acremont V, Hay Burgess DC, Hamer DH. Herlihy JM, et al. In: Black RE, Laxminarayan R, Temmerman M, Walker N, editors. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 Apr 5. Chapter 8. In: Black RE, Laxminarayan R, Temmerman M, Walker N, editors. Reproductive, Maternal, Newborn, and Child Health: Disease Control Priorities, Third Edition (Volume 2). Washington (DC): The International Bank for Reconstruction and Development / The World Bank; 2016 Apr 5. Chapter 8. PMID: 27227231 Free Books & Documents. Review. - Quantifying the contribution of Plasmodium falciparum malaria to febrile illness amongst African children.
Dalrymple U, Cameron E, Bhatt S, Weiss DJ, Gupta S, Gething PW. Dalrymple U, et al. Elife. 2017 Oct 16;6:e29198. doi: 10.7554/eLife.29198. Elife. 2017. PMID: 29034876 Free PMC article. - The relationship between reported fever and Plasmodium falciparum infection in African children.
Okiro EA, Snow RW. Okiro EA, et al. Malar J. 2010 Apr 19;9:99. doi: 10.1186/1475-2875-9-99. Malar J. 2010. PMID: 20398428 Free PMC article.
Cited by
- Host-Based Prognostic Biomarkers to Improve Risk Stratification and Outcome of Febrile Children in Low- and Middle-Income Countries.
Balanza N, Erice C, Ngai M, Varo R, Kain KC, Bassat Q. Balanza N, et al. Front Pediatr. 2020 Sep 18;8:552083. doi: 10.3389/fped.2020.552083. eCollection 2020. Front Pediatr. 2020. PMID: 33072673 Free PMC article. - Disparities between malaria infection and treatment rates: Evidence from a cross-sectional analysis of households in Uganda.
Saran I, Cohen J. Saran I, et al. PLoS One. 2017 Feb 27;12(2):e0171835. doi: 10.1371/journal.pone.0171835. eCollection 2017. PLoS One. 2017. PMID: 28241041 Free PMC article. - Millennium development health metrics: where do Africa's children and women of childbearing age live?
Tatem AJ, Garcia AJ, Snow RW, Noor AM, Gaughan AE, Gilbert M, Linard C. Tatem AJ, et al. Popul Health Metr. 2013 Jul 23;11(1):11. doi: 10.1186/1478-7954-11-11. Popul Health Metr. 2013. PMID: 23875684 Free PMC article. - Coverage of malaria protection in pregnant women in sub-Saharan Africa: a synthesis and analysis of national survey data.
van Eijk AM, Hill J, Alegana VA, Kirui V, Gething PW, ter Kuile FO, Snow RW. van Eijk AM, et al. Lancet Infect Dis. 2011 Mar;11(3):190-207. doi: 10.1016/S1473-3099(10)70295-4. Epub 2011 Jan 26. Lancet Infect Dis. 2011. PMID: 21273130 Free PMC article. - Aetiology of acute febrile episodes in children attending Korogwe District Hospital in north-eastern Tanzania.
Mahende C, Ngasala B, Lusingu J, Butichi A, Lushino P, Lemnge M, Premji Z. Mahende C, et al. PLoS One. 2014 Aug 4;9(8):e104197. doi: 10.1371/journal.pone.0104197. eCollection 2014. PLoS One. 2014. PMID: 25090651 Free PMC article.
References
- World Health Organization. Global Malaria Programme, WHO ACT policy update. Geneva: World Health Organization; 2009. Available: http://apps.who.int/malaria/amdp/amdp_afro.htm. Accessed 13 November 2009.
- World Health Organization. World malaria report 2009. Geneva: World Health Organization; 2009.
- Font F, Gonzalez MA, Nathan R, Kimario J, Lwilla F, et al. Diagnostic accuracy and case management of clinical malaria in the primary health services of a rural area in south-eastern Tanzania. Trop Med Int Health. 2001;6:423–428. - PubMed
- Kallander K, Nsungwa-Sabiiti J, Peterson S. Symptom overlap for malaria and pneumonia-policy implications for home management strategies. Acta Trop. 2004;90:211–214. - PubMed
- Chandramohan D, Jaffar S, Greenwood B. Use of clinical algorithms for diagnosing malaria. Trop Med Int Health. 2002;7:45–52. - PubMed
Publication types
MeSH terms
Grants and funding
- 079080/WT_/Wellcome Trust/United Kingdom
- 079091/WT_/Wellcome Trust/United Kingdom
- 081829/WT_/Wellcome Trust/United Kingdom
- WT_/Wellcome Trust/United Kingdom
- 086166/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Medical
Research Materials