Extensive analysis of D7S486 in primary gastric cancer supports TESTIN as a candidate tumor suppressor gene - PubMed (original) (raw)
Extensive analysis of D7S486 in primary gastric cancer supports TESTIN as a candidate tumor suppressor gene
Haiqing Ma et al. Mol Cancer. 2010.
Abstract
Background: High frequency of loss of heterozygosity (LOH) was found at D7S486 in primary gastric cancer (GC). And we found a high frequency of LOH region on 7q31 in primary GC from China, and identified D7S486 to be the most frequent LOH locus. This study was aimed to determine what genes were affected by the LOH and served as tumor suppressor genes (TSGs) in this region. Here, a high-throughput single nucleotide polymorphisms (SNPs) microarray fabricated in-house was used to analyze the LOH status around D7S486 on 7q31 in 75 patients with primary GC. Western blot, immunohistochemistry, and RT-PCR were used to assess the protein and mRNA expression of TESTIN (TES) in 50 and 140 primary GC samples, respectively. MTS assay was used to investigate the effect of TES overexpression on the proliferation of GC cell lines. Mutation and methylation analysis were performed to explore possible mechanisms of TES inactivation in GC.
Results: LOH analysis discovered five candidate genes (ST7, FOXP2, MDFIC, TES and CAV1) whose frequencies of LOH were higher than 30%. However, only TES showed the potential to be a TSG associated with GC. Among 140 pairs of GC samples, decreased TES mRNA level was found in 96 (68.6%) tumor tissues when compared with matched non-tumor tissues (p < 0.001). Also, reduced TES protein level was detected in 36 (72.0%) of all 50 tumor tissues by Western blot (p = 0.001). In addition, immunohistochemical staining result was in agreement with that of RT-PCR and Western blot. Down regulation of TES was shown to be correlated with tumor differentiation (p = 0.035) and prognosis (p = 0.035, log-rank test). Its overexpression inhibited the growth of three GC cell lines. Hypermethylation of TES promoter was a frequent event in primary GC and GC cell lines. However, no specific gene mutation was observed in the coding region of the TES gene.
Conclusions: Collectively, all results support the role of TES as a TSG in gastric carcinogenesis and that TES is inactivated primarily by LOH and CpG island methylation.
Figures
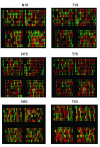
Figure 1
Representative results of microarray images from 3 primary GC samples by microdissection. "T" for tumor tissue and "N" for matched adjacent non-tumor tissue. (Each probe was printed twice and shown as neighboring spots. Spots in red and green, homozygous; yellow, heterozygous; and dark, low signal represented no signal or too low for genotype calls. White pane represented LOH, which is homozygous in tumor tissue but heterozygous in matched non-tumor tissue.)
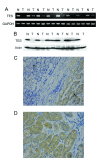
Figure 2
TES expression in primary GC. A. Representative results of TES mRNA expression in primary GC by RT-PCR. B. Representative results of TES protein expression in GC by Western blot. C. Representative staining of TES in GC tissue by immunohistochemistry (× 100). "T" and "N" represent tumor tissue and matched adjacent non-tumor tissue, respectively. D. Representative staining of TES in gastric non-tumor tissue (× 100).
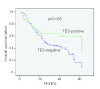
Figure 3
Estimated overall survival according to the expression of TES in 140 cases of primary GC (Kaplan-Meier method). The expression of TES was classified as negative expression (n = 108) and positive (n = 32) based on the immunohistochemical staining results.
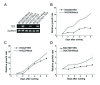
Figure 4
Overexpression of TES and its inhibitory effect on the proliferation of GC cells. Three GC cell lines used in this study were MGC803, HGC27 and SGC7901. A. TES mRNA expression in GC cells 36 h after transfection. B-D. The inhibitory effect of TES overexpression on the proliferation of GC cells sorted by flow cytometry for 7 consecutive days. This experiment is a representative of three independent experiments.
Figure 5
DNA Methylation analysis of TES promoter in GC. TES mRNA (A) and protein (B) expression were significantly down regulated in three GC cell lines (MGC803, HGC27 and SGC7901). Methylation analysis of TES promoter in three GC cell lines (C) and in 10 pairs of GC samples (D). Complete methylation was shown in MGC803 and HGC27 cells and in most GC samples. After DAC treatment, the complete methylation of TES promoter changed to partial methylation and nonmethylation in MGC803 and HGC27 cells, respectively (E). DAC treatment also reversed TES mRNA (F) and protein (G) expression in MGC803 and HGC27 cells. "M", "U" and"M-P"represent methylated CPG sequences, unmethylated CPG sequences and a positive control for methylation, respectively. "-" and "+" represent before and after DAC treatment, respectively.
Similar articles
- The Role of Testin in Human Cancers.
Popiel A, Kobierzycki C, Dzięgiel P. Popiel A, et al. Pathol Oncol Res. 2019 Oct;25(4):1279-1284. doi: 10.1007/s12253-018-0488-3. Epub 2018 Oct 25. Pathol Oncol Res. 2019. PMID: 30357755 Free PMC article. Review. - Extensive analysis of the 7q31 region in human prostate tumors supports TES as the best candidate tumor suppressor gene.
Chêne L, Giroud C, Desgrandchamps F, Boccon-Gibod L, Cussenot O, Berthon P, Latil A. Chêne L, et al. Int J Cancer. 2004 Sep 20;111(5):798-804. doi: 10.1002/ijc.20337. Int J Cancer. 2004. PMID: 15252854 - [Expression and clinical significance of TESTIN in primary gastric cancer].
Huang W, Weng DS, Pan ZZ, Pan K, Ding PR, Zhou J, Wang H, Zhang HK, Li JJ, Xia JC. Huang W, et al. Ai Zheng. 2008 Sep;27(9):984-8. Ai Zheng. 2008. PMID: 18799041 Chinese. - [Minimal commonly deleted regions on chromosome 7q31 in primary gastric carcinoma and its clinical significance].
Li JT, Fu L, Xia JC, Feng BJ, Mai SJ, Yu XJ, Huang LX, Feng QS, Pan ZZ, Zhan YQ. Li JT, et al. Ai Zheng. 2005 Nov;24(11):1306-11. Ai Zheng. 2005. PMID: 16552953 Chinese. - Involvement of the multiple tumor suppressor genes and 12-lipoxygenase in human prostate cancer. Therapeutic implications.
Gao X, Porter AT, Honn KV. Gao X, et al. Adv Exp Med Biol. 1997;407:41-53. doi: 10.1007/978-1-4899-1813-0_7. Adv Exp Med Biol. 1997. PMID: 9321930 Review.
Cited by
- TESTIN Induces Rapid Death and Suppresses Proliferation in Childhood B Acute Lymphoblastic Leukaemia Cells.
Weeks RJ, Ludgate JL, LeMée G, Morison IM. Weeks RJ, et al. PLoS One. 2016 Mar 17;11(3):e0151341. doi: 10.1371/journal.pone.0151341. eCollection 2016. PLoS One. 2016. PMID: 26985820 Free PMC article. - The Warburg effect dictates the mechanism of butyrate-mediated histone acetylation and cell proliferation.
Donohoe DR, Collins LB, Wali A, Bigler R, Sun W, Bultman SJ. Donohoe DR, et al. Mol Cell. 2012 Nov 30;48(4):612-26. doi: 10.1016/j.molcel.2012.08.033. Epub 2012 Oct 11. Mol Cell. 2012. PMID: 23063526 Free PMC article. - The PET and LIM1-2 domains of testin contribute to intramolecular and homodimeric interactions.
Sala S, Catillon M, Hadzic E, Schaffner-Reckinger E, Van Troys M, Ampe C. Sala S, et al. PLoS One. 2017 May 18;12(5):e0177879. doi: 10.1371/journal.pone.0177879. eCollection 2017. PLoS One. 2017. PMID: 28542564 Free PMC article. - Downregulation of TES by hypermethylation in glioblastoma reduces cell apoptosis and predicts poor clinical outcome.
Bai Y, Zhang QG, Wang XH. Bai Y, et al. Eur J Med Res. 2014 Dec 11;19(1):66. doi: 10.1186/s40001-014-0066-4. Eur J Med Res. 2014. PMID: 25498217 Free PMC article. - The Role of Testin in Human Cancers.
Popiel A, Kobierzycki C, Dzięgiel P. Popiel A, et al. Pathol Oncol Res. 2019 Oct;25(4):1279-1284. doi: 10.1007/s12253-018-0488-3. Epub 2018 Oct 25. Pathol Oncol Res. 2019. PMID: 30357755 Free PMC article. Review.
References
- Xia JC, Weng DS, Li JT, Qin HD, Mai SJ, Feng BJ, Fan Q, Feng QS, Huang LX, Yu XJ, Pan ZZ, Li YQ, Wang QJ, Zhan YQ, Chen SP, He J, Huang WL, Wu PH, Zeng YX. Loss of heterozygosity analysis of a candidate gastric carcinoma tumor suppressor locus at 7q31. Cancer Genet Cytogenet. 2006;166(2):166–172. doi: 10.1016/j.cancergencyto.2005.11.005. - DOI - PubMed
- Wang HY, Luo M, Tereshchenko IV, Frikker DM, Cui X, Li JY, Hu G, Chu Y, Azaro MA, Lin Y, Shen L, Yang Q, Kambouris ME, Gao R, Shih W, Li H. A genotyping system capable of simultaneously analyzing >1000 single nucleotide polymorphisms in a haploid genome. Genome Res. 2005;15(2):276–283. doi: 10.1101/gr.2885205. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous