MafA and MafB regulate genes critical to beta-cells in a unique temporal manner - PubMed (original) (raw)
. 2010 Oct;59(10):2530-9.
doi: 10.2337/db10-0190. Epub 2010 Jul 13.
Affiliations
- PMID: 20627934
- PMCID: PMC3279542
- DOI: 10.2337/db10-0190
MafA and MafB regulate genes critical to beta-cells in a unique temporal manner
Isabella Artner et al. Diabetes. 2010 Oct.
Abstract
Objective: Several transcription factors are essential to pancreatic islet β-cell development, proliferation, and activity, including MafA and MafB. However, MafA and MafB are distinct from others in regard to temporal and islet cell expression pattern, with β-cells affected by MafB only during development and exclusively by MafA in the adult. Our aim was to define the functional relationship between these closely related activators to the β-cell.
Research design and methods: The distribution of MafA and MafB in the β-cell population was determined immunohistochemically at various developmental and perinatal stages in mice. To identify genes regulated by MafB, microarray profiling was performed on wild-type and MafB(-/-) pancreata at embryonic day 18.5, with candidates evaluated by quantitative RT-PCR and in situ hybridization. The potential role of MafA in the expression of verified targets was next analyzed in adult islets of a pancreas-wide MafA mutant (termed MafA(ΔPanc)).
Results: MafB was produced in a larger fraction of β-cells than MafA during development and found to regulate potential effectors of glucose sensing, hormone processing, vesicle formation, and insulin secretion. Notably, expression from many of these genes was compromised in MafA(ΔPanc) islets, suggesting that MafA is required to sustain expression in adults.
Conclusions: Our results provide insight into the sequential manner by which MafA and MafB regulate islet β-cell formation and maturation.
Figures
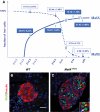
FIG. 1.
MafA and MafB expression is dynamically regulated in developing and adult β-cells. A: The percentage of β-cells coexpressing MafA or MafB was assessed at E14.5, E18.5, P14, and P28 by quantification of the number of insulin+ cells costaining with either MafA or MafB. Sections spanning the entire pancreas of wild-type mice were used; the results are presented as the mean ± SEM. B and C: MafB is sustained in a fraction of the insulin+ cells in MafA_Δ_panc islets. Wild-type (B) and mutant (C) pancreatic sections were stained for MafB (green) and insulin (red). Notably, MafB+ is not found in wild-type insulin+ cells, but is found in MafA_Δ_Panc. Yellow arrowhead denotes representative MafB+ insulin+ cells, and white arrowhead denotes MafB+ insulin− cells. Nuclei were stained with YO-PRO-1 in blue. MafB was found in 33.4 ± 5.6% of MafA_Δ_panc insulin+ cells (n = 3). (A high-quality digital representation of this figure is available in the online issue.)
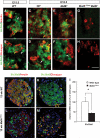
FIG. 2.
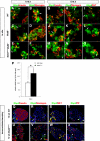
Islet zinc transporter Slc30a8 expression is activated by MafB during embryogenesis and by MafA in islet β-cells. Slc30a8 transcripts (green) were detected by in situ analysis in wild-type insulin+ (red, A and C) and glucagon+ (red, B and D) cells at E15.5 and E18.5. Expression is still detected in the remaining insulin+ cells (E), but lost in glucagon+ (F) cells in _MafB_−/− pancreata. No Slc30a8 expression was detected in the MafA and MafB compound mutant (MafA_Δ_Panc; _MafB_−/−; G and H). I: RT-PCR data illustrate the change in Slc30a8 mRNA expression between 3-month-old MafA_Δ_panc and wild-type islets. *P < 0.05 (n = 3). Slc30a8 was detected in islet β-cells (J) and α-cells (K) of 12-week-old wild-type islets by immunofluorescence. Expression was profoundly reduced in MafA_Δ_Panc β-cells (L) and unaffected in α-cells (M). The (L) Slc30a8+ insulin− cells labeled with white arrows correspond to (M) Slc30a8+ glucagon+ cells denoted by yellow arrows. Scale bar = 20 μm. Nuclei were stained in blue in panels J–M. (A high-quality digital representation of this figure is available in the online issue.)
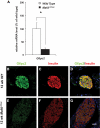
FIG. 3.
G6pc2 expression is compromised in adult islets lacking MafA. A: Quantitative RT-PCR analysis revealed that G6pc2 mRNA levels were significantly downregulated in adult MafA_Δ_Panc islets. *P < 0.05 (n = 3). G6pc2 expression (green) in islet insulin+ cells (B–D) (red) was shown by immunostaining to be profoundly reduced in MafA_Δ_panc islets (E–G). Scale bar = 20 μm. Nuclei were stained with YO-PRO-1 (blue). (A high-quality digital representation of this figure is available in the online issue.)
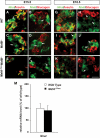
FIG. 4.
Nnat expression in β-cells is regulated only by MafB. Appreciable levels of Nnat (green) were detected in insulin+ (red, A, yellow arrows), insulin− (A, white arrows), but not glucagon+ (red, B) cells by in situ analysis. Nnat was essentially restricted to β-cells by E18.5 in the wild-type (G and H) and the remaining insulin+ cells in the _MafB_−/− and MafA_Δ_Panc _;MafB_−/− mutants (I and L). Scale bar = 20 μm. Nnat expression was unaffected in MafA_Δ_panc islets (M). Yellow arrows denote Nnat+hormone+ cells and white arrows denote Nnat+hormone− cells. (A high-quality digital representation of this figure is available in the online issue.)
FIG. 5.
Rbp4 is induced in the embryonic _MafB_−/− and islet MafA_Δ_Panc mutants. Rbp4 transcripts (green) are present in some (A and G) insulin+ (red, yellow arrows) and (B and H) glucagon+ (red) cells (A and B, yellow arrows) at both E15.5 and E18.5, with expression also found in (I) somatostatin+ δ-cells (red, yellow arrows) by E18.5. The number of hormone+ Rbp4+ cells increased in _MafB_−/− and MafA_Δ_Panc _;MafB_−/− mutant pancreata (C–F; J–O). P: Rbp4 mRNA expression was elevated in 12-week MafA_Δ_Panc islets. S–X: The Rbp4 protein is found in somatostatin (SST)+ δ-cells in (S) wild-type and (W) MafA_Δ_Panc islets, and not (Q and U) insulin+, (R and V) glucagon+, or (T and X) pancreatic peptide+ cells. Scale bar = 20 μm. Nuclei were stained in blue in panels Q–W. White arrows denote Rbp4+ hormone− cells. (A high-quality digital representation of this figure is available in the online issue.)
Similar articles
- A switch from MafB to MafA expression accompanies differentiation to pancreatic beta-cells.
Nishimura W, Kondo T, Salameh T, El Khattabi I, Dodge R, Bonner-Weir S, Sharma A. Nishimura W, et al. Dev Biol. 2006 May 15;293(2):526-39. doi: 10.1016/j.ydbio.2006.02.028. Epub 2006 Apr 3. Dev Biol. 2006. PMID: 16580660 Free PMC article. - The MafA transcription factor becomes essential to islet β-cells soon after birth.
Hang Y, Yamamoto T, Benninger RK, Brissova M, Guo M, Bush W, Piston DW, Powers AC, Magnuson M, Thurmond DC, Stein R. Hang Y, et al. Diabetes. 2014 Jun;63(6):1994-2005. doi: 10.2337/db13-1001. Epub 2014 Feb 11. Diabetes. 2014. PMID: 24520122 Free PMC article. - MafA and MafB activity in pancreatic β cells.
Hang Y, Stein R. Hang Y, et al. Trends Endocrinol Metab. 2011 Sep;22(9):364-73. doi: 10.1016/j.tem.2011.05.003. Epub 2011 Jun 28. Trends Endocrinol Metab. 2011. PMID: 21719305 Free PMC article. Review. - Differentiation of pancreatic endocrine progenitors reversibly blocked by premature induction of MafA.
He KH, Juhl K, Karadimos M, El Khattabi I, Fitzpatrick C, Bonner-Weir S, Sharma A. He KH, et al. Dev Biol. 2014 Jan 1;385(1):2-12. doi: 10.1016/j.ydbio.2013.10.024. Epub 2013 Oct 29. Dev Biol. 2014. PMID: 24183936 Free PMC article. - Exploring Large MAF Transcription Factors: Functions, Pathology, and Mouse Models with Point Mutations.
Fujino M, Ojima M, Takahashi S. Fujino M, et al. Genes (Basel). 2023 Sep 27;14(10):1883. doi: 10.3390/genes14101883. Genes (Basel). 2023. PMID: 37895232 Free PMC article. Review.
Cited by
- Superior beta cell proliferation, function and gene expression in a subpopulation of rat islets identified by high blood perfusion.
Lau J, Svensson J, Grapensparr L, Johansson Å, Carlsson PO. Lau J, et al. Diabetologia. 2012 May;55(5):1390-9. doi: 10.1007/s00125-012-2476-6. Epub 2012 Feb 5. Diabetologia. 2012. PMID: 22311418 - Notch signaling dynamically regulates adult β cell proliferation and maturity.
Bartolome A, Zhu C, Sussel L, Pajvani UB. Bartolome A, et al. J Clin Invest. 2019 Jan 2;129(1):268-280. doi: 10.1172/JCI98098. Epub 2018 Dec 3. J Clin Invest. 2019. PMID: 30375986 Free PMC article. - Thioredoxin-interacting protein regulates insulin transcription through microRNA-204.
Xu G, Chen J, Jing G, Shalev A. Xu G, et al. Nat Med. 2013 Sep;19(9):1141-6. doi: 10.1038/nm.3287. Epub 2013 Aug 25. Nat Med. 2013. PMID: 23975026 Free PMC article. - Glucose regulates MafA transcription factor abundance and insulin gene expression by inhibiting AMP-activated protein kinase in pancreatic β-cells.
Iwaoka R, Kataoka K. Iwaoka R, et al. J Biol Chem. 2018 Mar 9;293(10):3524-3534. doi: 10.1074/jbc.M117.817932. Epub 2018 Jan 18. J Biol Chem. 2018. PMID: 29348175 Free PMC article. Retracted. - Reprogramming of human pancreatic exocrine cells to β-like cells.
Lemper M, Leuckx G, Heremans Y, German MS, Heimberg H, Bouwens L, Baeyens L. Lemper M, et al. Cell Death Differ. 2015 Jul;22(7):1117-30. doi: 10.1038/cdd.2014.193. Epub 2014 Dec 5. Cell Death Differ. 2015. PMID: 25476775 Free PMC article.
References
- Gradwohl G. Development of the endocrine pancreas. Diabete Metab 2006;32:532–533 - PubMed
- Kim SK, MacDonald RJ. Signaling and transcriptional control of pancreatic organogenesis. Curr Opin Genet Dev 2002;12:540–547 - PubMed
- D'Amour KA, Bang AG, Eliazer S, Kelly OG, Agulnick AD, Smart NG, Moorman MA, Kroon E, Carpenter MK, Baetge EE. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol 2006;24:1392–1401 - PubMed
- Kroon E, Martinson LA, Kadoya K, Bang AG, Kelly OG, Eliazer S, Young H, Richardson M, Smart NG, Cunningham J, Agulnick AD, D'Amour KA, Carpenter MK, Baetge EE. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol 2008;26:443–452 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P60 DK020593/DK/NIDDK NIH HHS/United States
- P01 DK-42502/DK/NIDDK NIH HHS/United States
- P60 DK-20593/DK/NIDDK NIH HHS/United States
- U01 DK072473/DK/NIDDK NIH HHS/United States
- DK-72433/DK/NIDDK NIH HHS/United States
- R01 DK072433/DK/NIDDK NIH HHS/United States
- P01 DK042502/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases