Transitions between male and female heterogamety caused by sex-antagonistic selection - PubMed (original) (raw)
Transitions between male and female heterogamety caused by sex-antagonistic selection
G Sander van Doorn et al. Genetics. 2010 Oct.
Abstract
Many animal taxa show frequent and rapid transitions between male heterogamety (XY) and female heterogamety (ZW). We develop a model showing how these transitions can be driven by sex-antagonistic selection. Sex-antagonistic selection acting on loci linked to a new sex-determination mutation can cause it to invade, but when acting on loci linked to the ancestral sex-determination gene will inhibit an invasion. The strengths of the consequent indirect selection on the old and new sex-determination loci are mediated by the strengths of sex-antagonistic selection, linkage between the sex-antagonistic and sex-determination genes, and the amount of genetic variation. Sex-antagonistic loci that are tightly linked to a sex-determining gene have a vastly stronger influence on the balance of selection than more distant loci. As a result, changes in linkage, caused, for example, by an inversion that captures a sex-determination mutation and a gene under sex-antagonistic selection, can trigger transitions between XY and ZW systems. Sex-antagonistic alleles can become more strongly associated with pleiotropically dominant sex-determining factors, which may help to explain biases in the rates of transitions between male and female heterogamety. Deleterious recessive mutations completely linked to the ancestral Y chromosome can prevent invasion of a neo-W chromosome or result in a stable equilibrium at which XY and ZW systems segregate simultaneously at two linkage groups.
Figures
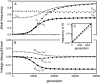
Figure 1.—
A homologous transition from male to female heterogamety. (A) A feminizing allele segregating at a novel sex-determination locus on the ancestral sex chromosomes increases in frequency (solid circles) if it is more tightly linked to a sex-antagonistic locus than the ancestral master sex-determining gene ( and
in this simulation). The spread of the novel W allele is accompanied by an increase in the frequency of the ancestral masculinizing Y allele in both male and female gametes (open and solid squares, respectively) and slight changes in the allele frequencies at the sex-antagonistic locus (open and solid triangles/dashed lines). (B) The homologous heterogamety transition is driven by sex-antagonistic selection, which acts indirectly on the sex-determination loci through their nonrandom genetic association with sex-antagonistic alleles. (C) Initially, the W allele spreads at a nearly constant exponential rate and simulated frequencies (solid circles) fall along a straight line when plotted on a logarithmic scale. Analytical expressions for the invasion fitness of the W allele (dashed line, loose-linkage result; solid line, tight-linkage result) predict the slope of this line (i.e., the exponential rate of increase) during the initial phase of the simulations. Fitness values for the genotypes 00, 01, and 11 in females were given by
,
, and
, respectively, with
and
. Analogous expressions define fitness in males, with
and
. Mutations between sex-antagonistic alleles occurred at rate
.
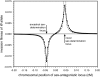
Figure 2.—
Validity of the analytical approximations. A homologous transition is modeled with a fixed distance between the ancestral and novel sex-determining loci (the rate of recombination between these loci is ), while the position of the sex-antagonistic locus (parameters:
,
,
,
, and
) is varied (horizontal axis). The vertical axis gives the invasion fitness of the W allele on the basis of numerical iterations of the exact population-genetic recursions of the model (solid circles), the tight-linkage approximation (solid curve), and the loose-linkage approximation (dashed curve). The latter performs well as long as recombination is a stronger force than selection (
), while the tight-linkage approximation is accurate over the entire range of recombination rates (noticeable differences with the simulation results appear only when linkage between the sex-determination loci is very tight). We assumed H
aldane
's (1919) mapping function.
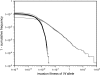
Figure 3.—
Heterogamety transitions are governed by a small subset of the sex-antagonistic genes. The invasion fitness of the W allele was calculated for 300,000 random genetic systems, each with 200 sex-antagonistic loci. In each simulation the sex-antagonistic loci were distributed over two linkage groups; two-thirds of the loci were randomly positioned on the ancestral sex chromosomes and the remaining one-third of the loci were randomly positioned on an autosome pair with the novel sex-determination gene. The size of both linkage groups was 100 cM. Despite the twofold bias favoring the ancestral sex-determination system and the large number of loci, we observed positive values of the invasion fitness in ∼10% of the cases. The right tail of the observed distribution of fitness values is shown, with dots indicating invasion fitness estimates from individual simulations (shaded dots, loose-linkage approximation; solid dots, tight-linkage approximation). In both cases, the right-tail behavior is well approximated by the fitness contribution of the single sex-antagonistic locus that is most tightly linked to the novel sex-determination gene (thin solid lines), indicating that the sex-antagonistic variation responsible for transitions between sex-determination systems may segregate at a very small subset of the sex-antagonistic loci. The selection coefficients for individual loci were drawn from a bivariate normal distribution with mean (0, 0) and a covariance matrix with common variance and correlation coefficient −0.8. The dominance coefficients were also drawn from a bivariate normal distribution [mean (0.5, 0.5), common variance
, and correlation coefficient 0.7] with the additional constraint 0 ≤ h ≤ 1.
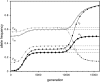
Figure 4.—
Protected polymorphism of two sex-determination factors on different linkage groups. Recessive deleterious alleles on the ancestral y chromosome can prevent the W allele from spreading to fixation, leading to a permanent state of multifactorial sex determination (equilibrium frequencies indicated by the dashed lines) if the deleterious alleles have fixed on the y chromosome and recombination with the x has been fully repressed. Even a tiny amount of recombination ( here), however, introduces genetic variation on the y chromosome and purges its deleterious alleles. When this happens, W ultimately fixes and the ancestral XY system is lost. In this simulation, a locus segregating for deleterious alleles (parameters:
,
,
, and deleterious mutation rate
; allele frequencies in male and female gametes are shown by dotted lines with open and solid triangles, respectively) is linked to Y and an autosomal sex-antagonistic locus (parameters:
,
,
,
,
, and
; allele frequencies are not shown) is linked to W.
Similar articles
- Impact of deleterious mutations, sexually antagonistic selection, and mode of recombination suppression on transitions between male and female heterogamety.
Saunders PA, Neuenschwander S, Perrin N. Saunders PA, et al. Heredity (Edinb). 2019 Sep;123(3):419-428. doi: 10.1038/s41437-019-0225-z. Epub 2019 Apr 27. Heredity (Edinb). 2019. PMID: 31028370 Free PMC article. - Haploid selection, sex ratio bias, and transitions between sex-determining systems.
Scott MF, Osmond MM, Otto SP. Scott MF, et al. PLoS Biol. 2018 Jun 25;16(6):e2005609. doi: 10.1371/journal.pbio.2005609. eCollection 2018 Jun. PLoS Biol. 2018. PMID: 29940019 Free PMC article. - Transitions in sex determination mechanisms through parental and sexual antagonism.
Schenkel MA. Schenkel MA. Heredity (Edinb). 2024 Nov;133(5):331-341. doi: 10.1038/s41437-024-00717-x. Epub 2024 Aug 21. Heredity (Edinb). 2024. PMID: 39164521 - Patterns and mechanisms of evolutionary transitions between genetic sex-determining systems.
Sander van Doorn G. Sander van Doorn G. Cold Spring Harb Perspect Biol. 2014 Jul 3;6(8):a017681. doi: 10.1101/cshperspect.a017681. Cold Spring Harb Perspect Biol. 2014. PMID: 24993578 Free PMC article. Review. - The Evolution of Genome Structure by Natural and Sexual Selection.
Kirkpatrick M. Kirkpatrick M. J Hered. 2017 Jan;108(1):3-11. doi: 10.1093/jhered/esw041. Epub 2016 Jul 7. J Hered. 2017. PMID: 27388336 Free PMC article. Review.
Cited by
- Sex chromosomes in the tribe Cyprichromini (Teleostei: Cichlidae) of Lake Tanganyika.
Behrens KA, Koblmüller S, Kocher TD. Behrens KA, et al. Sci Rep. 2022 Oct 26;12(1):17998. doi: 10.1038/s41598-022-23017-y. Sci Rep. 2022. PMID: 36289404 Free PMC article. - Males That Silence Their Father's Genes: Genomic Imprinting of a Complete Haploid Genome.
de la Filia AG, Mongue AJ, Dorrens J, Lemon H, Laetsch DR, Ross L. de la Filia AG, et al. Mol Biol Evol. 2021 May 19;38(6):2566-2581. doi: 10.1093/molbev/msab052. Mol Biol Evol. 2021. PMID: 33706381 Free PMC article. - Characterization of a large sex determination region in Salix purpurea L. (Salicaceae).
Zhou R, Macaya-Sanz D, Rodgers-Melnick E, Carlson CH, Gouker FE, Evans LM, Schmutz J, Jenkins JW, Yan J, Tuskan GA, Smart LB, DiFazio SP. Zhou R, et al. Mol Genet Genomics. 2018 Dec;293(6):1437-1452. doi: 10.1007/s00438-018-1473-y. Epub 2018 Jul 18. Mol Genet Genomics. 2018. PMID: 30022352 - Sex Differences in Recombination in Sticklebacks.
Sardell JM, Cheng C, Dagilis AJ, Ishikawa A, Kitano J, Peichel CL, Kirkpatrick M. Sardell JM, et al. G3 (Bethesda). 2018 May 31;8(6):1971-1983. doi: 10.1534/g3.118.200166. G3 (Bethesda). 2018. PMID: 29632132 Free PMC article. - Conservation of Sex-Linked Markers among Conspecific Populations of a Viviparous Skink, Niveoscincus ocellatus, Exhibiting Genetic and Temperature-Dependent Sex Determination.
Hill PL, Burridge CP, Ezaz T, Wapstra E. Hill PL, et al. Genome Biol Evol. 2018 Apr 1;10(4):1079-1087. doi: 10.1093/gbe/evy042. Genome Biol Evol. 2018. PMID: 29659810 Free PMC article.
References
- Bull, J. J., 1983. Evolution of Sex Determining Mechanisms. Benjamin/Cummings, Reading, MA.
- Bull, J. J., and E. L. Charnov, 1977. Changes in the heterogametic mechanism of sex determination. Heredity 39 1–14. - PubMed
- Charlesworth, B., 1991. The evolution of sex chromosomes. Science 251 1030–1033. - PubMed
- Charlesworth, B., and D. Charlesworth, 1978. A model for the evolution of dioecy and gynodioecy. Am. Nat. 112 975–997.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials