Visualizing the distribution of synapses from individual neurons in the mouse brain - PubMed (original) (raw)
Visualizing the distribution of synapses from individual neurons in the mouse brain
Ling Li et al. PLoS One. 2010.
Abstract
Background: Proper function of the mammalian brain relies on the establishment of highly specific synaptic connections among billions of neurons. To understand how complex neural circuits function, it is crucial to precisely describe neuronal connectivity and the distributions of synapses to and from individual neurons.
Methods and findings: In this study, we present a new genetic synaptic labeling method that relies on expression of a presynaptic marker, synaptophysin-GFP (Syp-GFP) in individual neurons in vivo. We assess the reliability of this method and use it to analyze the spatial patterning of synapses in developing and mature cerebellar granule cells (GCs). In immature GCs, Syp-GFP is distributed in both axonal and dendritic regions. Upon maturation, it becomes strongly enriched in axons. In mature GCs, we analyzed synapses along their ascending segments and parallel fibers. We observe no differences in presynaptic distribution between GCs born at different developmental time points and thus having varied depths of projections in the molecular layer. We found that the mean densities of synapses along the parallel fiber and the ascending segment above the Purkinje cell (PC) layer are statistically indistinguishable, and higher than previous estimates. Interestingly, presynaptic terminals were also found in the ascending segments of GCs below and within the PC layer, with the mean densities two-fold lower than that above the PC layer. The difference in the density of synapses in these parts of the ascending segment likely reflects the regional differences in postsynaptic target cells of GCs.
Conclusions: The ability to visualize synapses of single neurons in vivo is valuable for studying synaptogenesis and synaptic plasticity within individual neurons as well as information flow in neural circuits.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
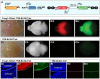
Figure 1. Strategy for creating a transgenic tripartite system for presynaptic labeling in vivo.
(A) Schematic representation of the three transgenes used for in vivo synaptic labeling: A ubiquitous (pU) or tissue-specific (pS) promoter driven Cre/CreER transgene (left); A Cre/CreER-dependent tTA knock-in (ZtTA) targeted to the ROSA26 locus (middle); A bidirectional TRE (TRE-Bi) transgene driving Synaptophysin-GFP (Syp-GFP) and tdT (TRE-Bi-SG-T) (right). I, chicken β-globin insulators were present in one version of this transgene (iiTRE-Bi-SG-Tii). (B–E) Proof-of-principle experiments showing tTA-dependent expression of Syp-GFP and tdT. (B) Whole-mount brain images of a double transgenic mouse (Foxg1-tTA/wt; TRE-Bi-SG-T/wt). (C) Whole mount brain images of a control mouse (TRE-Bi-SG-T/wt). Bii and Cii, bright field images; Biii and Ciii, tdT expression; Biv and Civ, Syp-GFP expression. (D) Confocal image of a brain section from a P7 double transgenic mouse (Foxg1-tTA/wt; TRE-Bi-SG-T/wt) showing that expression of the TRE-Bi transgene is restricted to forebrain. (E) Confocal image of a brain section from a P7 control mouse (TRE-Bi-SG-T/wt) showing that the TRE-Bi transgene alone is not expressed when a tTA transgene is absent. Di and Ei, DAPI; Dii and Eii, tdT; Diii and Eiii, Syp-GFP. White dashed line demarks the forebrain-hindbrain boundary. Scale bar = 50 µm.
Figure 2. Dox-dependent gene activation.
(A–E) Representative confocal images of labeled cerebellar GCs from P30 triple transgenic mouse (ZtTA/wt; TRE-Bi-SG-T/wt; β-actin-CreER/wt). Tamoxifen was injected at P7 and Dox was never administered (E) or it was removed for 0 (A), 3 (B), 7 (C) or 14 (D) days. Scale bar = 25 µm. (F) Diagram of the experimental procedure described above. (G) Quantification of the average fluorescence intensity in cerebellar GC bodies indicates that the expression level of the TRE-driven transgene increases the earlier Dox is removed, reaching maximal expression by 14 days after Dox removal. Error bars represent standard deviation. “n” represents the number of analyzed cell bodies for each condition indicated. Two animals per Dox condition were used for analysis. (H) Quantification of the total number of labeled cerebellar GCs per 225×225 µm2 for each Dox condition shows no statistically significant differences between different conditions. Error bars represent standard deviation.
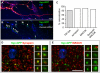
Figure 3. Validation of the synaptic labeling tool.
(A) A representative image showing functional presynaptic terminals labeled by FM dye staining (blue) in 14 DIV (days in vitro) dissociated primary hippocampal neurons generated from a P0 triple transgenic mouse pup (ZtTA/wt; TRE-Bi-SG-T/wt; Nestin-Cre/wt). The presynaptic termini, labeled by Syp-GFP (green) and the FM dye (blue) are located on axonal processes (red) and therefore appear white or yellowish. (B) The Syp-GFP and FM-dye channels from panel A have been shifted to show that most Syp-GFP puncta (green, white arrowheads) located along the processes colocalize with FM dye (blue) puncta. Scale bar is 5 µm. (C) Quantification of percentage of Syp-GFP colocalized with FM dye (Grey bar for FM dye experiment in A–B), synapsin and MAGUK (for array tomography experiment in D–E). The total number of counted Syp-GFP puncta is 1176 and 314, for the FM dye experiment and array tomography experiment, respectively. Two animals were used for the FM dye experiment. (D–E) Representative array tomography images from a P35 triple transgenic mouse brain (ZtTA/wt; TRE-Bi-SG-T/wt; β-actin-CreER/wt) with tamoxifen administered at E9.5 showing in vivo presynaptic localization of Syp-GFP puncta. Left panels represent images from a single ultrathin (70 nm) section showing colocalization of Syp-GFP (green) with synapsin (red, C) or MAGUK (red, D). Right panels show two examples (each example in a single column) of 4 serial sections through a single Syp-GFP punctum.
Figure 4. Quantitative analysis of Syp-GFP distribution in cerebellar granule cells.
(A) Schematic illustration of the organization and major neuronal types of the cerebellar cortex. ML, molecular layer; PC, Purkinje cell body layer; GL, granular layer; WM, white matter. (B) Representative confocal image of a labeled mature cerebellar GC in a ZtTA/wt; TRE-Bi-SG-T/wt; β-actin-CreER/wt mouse with tamoxifen administered at P7 and dissected at P30, 7 days after Dox removal. The overlap between Syp-GFP puncta and tdT labeled GC processes is evident in the merged image, where Syp-GFP puncta appear yellow. Cerebellar layers (as illustrated in panel A) are marked with white lines. Scale bar = 20 µm. The last panel shows a snapshot of the same cerebellar GC after filament and puncta tracing in Imaris. (C) Representative confocal image of labeled parallel fibers and a snapshot of a single traced parallel fiber (bifurcation point marked by a white arrow in the upper image). (D) Quantification of Syp-GFP mean density (the number of puncta per 100 µm) for different populations of GCs (N = 6 for each population, except N = 5 for the deep sublayer ascending segment above PC layer). We quantified the density of Syp-GFP puncta in the ascending segment below the PC layer (Below PC), within the PC layer (PC), above the PC layer (Above PC) and in the parallel fibers (PF) for GCs projecting their axons to superficial (S), middle (M) or deep (D) regions of the molecular layer. Each green dot represents a data point. A black line marks the mean of each column. Error bars are ± standard error of the mean (SEM). Six animals were used for analysis. (E) Plot showing the Syp-GFP puncta distribution along linearized ascending segments of each traced GC. The light brown shading represents the Purkinje cell layer. Red solid squares on the left represent the locations where individual ascending axons initiate from corresponding cell bodies. Red empty squares represent the locations where an ascending axon initiates from a dendrite. The open blue circle on the right represents the bifurcation point without a Syp-GFP punctum. Blue dots on the right represent the bifurcation points containing a Syp-GFP punctum. Numbers below the plots represent distance in µm. Cells are aligned where the PC layer starts; to the left below the PC layer, and to the right above the PC layer. (F) A plot showing the distribution of Syp-GFP puncta along linearized parallel fibers of traced GCs. The blue vertical line marks the bifurcation points. Numbers below the plots represent distance in µm. Axons are aligned at the bifurcation point.
Figure 5. Syp-GFP distribution patterns in developing and mature GCs.
(A) Representative confocal image of a labeled immature cerebellar GC from the mouse with the following genotype: ZtTA/wt; TRE-Bi-SG-T/wt; β-actin-CreER/wt, with tamoxifen administered at P7, and dissected at P15. In the merged panel Syp-GFP appears yellow, while tdT (red) fills the processes. (B) Quantification of Syp-GFP distribution in axonal and dendritic regions in mature and immature GCs showing that Syp-GFP becomes enriched in the axon after maturation. The graph shows an average for 10 cells for each condition. For mature cells, the standard deviation is ±11%; for immature cells, the standard deviation is ±7%. Two animals were used for analysis.
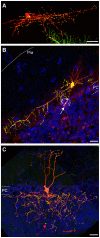
Figure 6. Morphology and presynaptic distribution in cerebellar interneurons.
Representative images of Syp-GFP distribution in a cerebellar stellate cell (A), basket cell (B) and Golgi cell (C). Syp-GFP (green), tdT (red), TOTO3 (blue). White dashed lines (B) highlight the PC layer (estimated by the TOTO3 signal of PC bodies) and the pial surface. Arrow points to a “basket.” Labeled GC axons in (B) were removed for presentation purposes using Photoshop. White dashed lines (C) highlight the PC layer in the cerebellum (estimated by the TOTO3 signal of PC bodies). All cells are from triple transgenic mice (ZtTA/wt; TRE-Bi-SG-T/wt; β-actin-CreER/wt) dissected at P21 (A), P30 (B) and P15 (C). Tamoxifen was administered at E9.5 (A), P7 (B) and E17.5 (C). Scale bars are 20 µm.
Similar articles
- Specific labeling of climbing fibers shows early synaptic interactions with immature Purkinje cells in the prenatal cerebellum.
Kita Y, Tanaka K, Murakami F. Kita Y, et al. Dev Neurobiol. 2015 Sep;75(9):927-34. doi: 10.1002/dneu.22259. Epub 2014 Dec 24. Dev Neurobiol. 2015. PMID: 25529108 - Balanced GABAergic and glutamatergic synapse development in hippocampal neurons.
Zhao X, Shoji S, Lau P. Zhao X, et al. Biochem Biophys Res Commun. 2005 May 20;330(4):1110-5. doi: 10.1016/j.bbrc.2005.03.083. Biochem Biophys Res Commun. 2005. PMID: 15823558 - The actin-binding protein profilin I is localized at synaptic sites in an activity-regulated manner.
Neuhoff H, Sassoè-Pognetto M, Panzanelli P, Maas C, Witke W, Kneussel M. Neuhoff H, et al. Eur J Neurosci. 2005 Jan;21(1):15-25. doi: 10.1111/j.1460-9568.2004.03814.x. Eur J Neurosci. 2005. PMID: 15654839 - [Glutamate signaling and neural plasticity].
Watanabe M. Watanabe M. No To Hattatsu. 2013 Jul;45(4):267-74. No To Hattatsu. 2013. PMID: 23951937 Review. Japanese. - Glutamate-receptor-like molecule GluRδ2 involved in synapse formation at parallel fiber-Purkinje neuron synapses.
Hirano T. Hirano T. Cerebellum. 2012 Mar;11(1):71-7. doi: 10.1007/s12311-010-0170-0. Cerebellum. 2012. PMID: 20387025 Review.
Cited by
- Cerebellar output in zebrafish: an analysis of spatial patterns and topography in eurydendroid cell projections.
Heap LA, Goh CC, Kassahn KS, Scott EK. Heap LA, et al. Front Neural Circuits. 2013 Apr 1;7:53. doi: 10.3389/fncir.2013.00053. eCollection 2013. Front Neural Circuits. 2013. PMID: 23554587 Free PMC article. - Q&A: Array tomography.
Smith SJ. Smith SJ. BMC Biol. 2018 Sep 6;16(1):98. doi: 10.1186/s12915-018-0560-1. BMC Biol. 2018. PMID: 30189863 Free PMC article. - Modernization of Golgi staining techniques for high-resolution, 3-dimensional imaging of individual neurons.
Vints K, Vandael D, Baatsen P, Pavie B, Vernaillen F, Corthout N, Rybakin V, Munck S, Gounko NV. Vints K, et al. Sci Rep. 2019 Jan 15;9(1):130. doi: 10.1038/s41598-018-37377-x. Sci Rep. 2019. PMID: 30644431 Free PMC article. - A medullary centre for lapping in mice.
Dempsey B, Sungeelee S, Bokiniec P, Chettouh Z, Diem S, Autran S, Harrell ER, Poulet JFA, Birchmeier C, Carey H, Genovesio A, McMullan S, Goridis C, Fortin G, Brunet JF. Dempsey B, et al. Nat Commun. 2021 Nov 2;12(1):6307. doi: 10.1038/s41467-021-26275-y. Nat Commun. 2021. PMID: 34728601 Free PMC article. - Large-scale automated histology in the pursuit of connectomes.
Kleinfeld D, Bharioke A, Blinder P, Bock DD, Briggman KL, Chklovskii DB, Denk W, Helmstaedter M, Kaufhold JP, Lee WC, Meyer HS, Micheva KD, Oberlaender M, Prohaska S, Reid RC, Smith SJ, Takemura S, Tsai PS, Sakmann B. Kleinfeld D, et al. J Neurosci. 2011 Nov 9;31(45):16125-38. doi: 10.1523/JNEUROSCI.4077-11.2011. J Neurosci. 2011. PMID: 22072665 Free PMC article. Review.
References
- Shepherd GM. The Synaptic Organization of the Brain. Oxford University Press; 2004.
- Tang SH, Silva FJ, Tsark WM, Mann JR. A Cre/loxP-deleter transgenic line in mouse strain 129S1/SvImJ. Genesis. 2002;32:199–202. - PubMed
- Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L. Mosaic analysis with double markers in mice. Cell. 2005;121:479–492. - PubMed
- Guo C, Yang W, Lobe CG. A Cre recombinase transgene with mosaic, widespread tamoxifen-inducible action. Genesis. 2002;32:8–18. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 DC008277/DC/NIDCD NIH HHS/United States
- R01 NS050835/NS/NINDS NIH HHS/United States
- HHMI/Howard Hughes Medical Institute/United States
- R01-NS050835/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous