The metabolic life and times of a T-cell - PubMed (original) (raw)
Review
The metabolic life and times of a T-cell
Ryan D Michalek et al. Immunol Rev. 2010 Jul.
Abstract
The regulation of lymphocyte homeostasis is critical for the development and formation of productive immune responses. Cell numbers must be maintained to allow sufficient numbers of lymphocytes to combat foreign pathogens but prevent the accumulation of excess lymphocytes that may increase the risk of developing autoimmunity or neoplasia. Cell extrinsic growth factors are essential to maintain homeostasis and cell survival, and it has become increasingly apparent that a key mechanism of this control is through regulation of cell metabolism. The metabolic state of T cells can have profound influences on cell growth and survival and even differentiation. In particular, resting T cells utilize an energy efficient oxidative metabolism but shift to a highly glycolytic metabolism when stimulated to grow and proliferate by pathogen encounter. After antigen clearance, T cells must return to a more quiescent oxidative metabolism to support T-cell memory. This review highlights how these metabolic changes may be intricately involved with both T-cell growth and death in the control of homeostasis and immunity.
Figures
Fig. 1. T cells transition to from oxidative to glycolytic metabolism during an immune response and return to oxidation for memory
Naive quiescent T cells meet basal energy demands through the oxidation of glucose and glutamine in the mitochondria to yield maximal ATP production. Upon activation, naive lymphocytes shift from a resting to highly proliferative and functional effector state. This transition requires a massive increase in glycolytic metabolism characterized by T-cell receptor (TCR) and costimulatory receptor (CD28)-stimulated expression of the glucose transporter Glut1, Akt-dependent trafficking of Glut1 to the cell surface, and increased glucose uptake. Following pathogen clearance, surviving effector cells differentiate into long-lived memory cells and revert to an oxidative metabolic state that is characterized by lipid oxidation and promoted by TRAF6, AMPK, and mTOR.
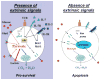
Fig. 2. T cells require extrinsic signals for metabolic maintenance and survival
T cells utilize common γ-chain cytokine signals (i.e. IL-7R, IL-2R) to promote Akt-dependent trafficking of Glut1 to the cell surface to promote glucose uptake and to stabilize expression of anti-apoptotic proteins (i.e. Bcl-2, Mcl-1). Furthermore, growth signals from the TCR also stimulate expression of Glut1 and contribute to increased glycolytic and mitochondrial metabolism. In the absence of these extrinsic signals, T cells fail to maintain glycolytic and mitochondrial metabolism as Glut1 becomes internalized and trafficked to lysosomes. Anti-apoptotic protein expression decreases while pro-apoptotic protein expression increases (i.e. Bim, Puma), sensitizing to programmed cell death.
References
- Cham CM, Gajewski TF. Glucose availability regulates IFN-gamma production and p70S6 kinase activation in CD8+ effector T cells. J Immunol. 2005;174:4670–4677. - PubMed
- Rathmell JC, Elstrom RL, Cinalli RM, Thompson CB. Activated Akt promotes increased resting T cell size, CD28-independent T cell growth, and development of autoimmunity and lymphoma. Eur J Immunol. 2003;33:2223–2232. - PubMed
- Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002;2:547–556. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources