B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity - PubMed (original) (raw)
Thomas Prod'homme, Juan C Patarroyo, Nicolas Molnarfi, Tara Karnezis, Klaus Lehmann-Horn, Dimitry M Danilenko, Jeffrey Eastham-Anderson, Anthony J Slavin, Christopher Linington, Claude C A Bernard, Flavius Martin, Scott S Zamvil
Affiliations
- PMID: 20641064
- PMCID: PMC3375897
- DOI: 10.1002/ana.22081
B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity
Martin S Weber et al. Ann Neurol. 2010 Sep.
Abstract
Objective: Clinical studies indicate that anti-CD20 B-cell depletion may be an effective multiple sclerosis (MS) therapy. We investigated mechanisms of anti-CD20-mediated immune modulation using 2 paradigms of experimental autoimmune encephalomyelitis (EAE).
Methods: Murine EAE was induced by recombinant myelin oligodendrocyte glycoprotein (rMOG), a model in which B cells are considered to contribute pathogenically, or MOG peptide (p)35-55, which does not require B cells.
Results: In EAE induced by rMOG, B cells became activated and, when serving as antigen-presenting cells (APCs), promoted differentiation of proinflammatory MOG-specific Th1 and Th17 cells. B-cell depletion prevented or reversed established rMOG-induced EAE, which was associated with less central nervous system (CNS) inflammation, elimination of meningeal B cells, and reduction of MOG-specific Th1 and Th17 cells. In contrast, in MOG p35-55-induced EAE, B cells did not become activated or efficiently polarize proinflammatory MOG-specific T cells, similar to naive B cells. In this setting, anti-CD20 treatment exacerbated EAE, and did not impede development of Th1 or Th17 cells. Irrespective of the EAE model used, B-cell depletion reduced the frequency of CD4(+)CD25(+)Foxp3(+) regulatory T cells (Treg), and increased the proinflammatory polarizing capacity of remaining myeloid APCs.
Interpretation: Our study highlights distinct roles for B cells in CNS autoimmunity. Clinical benefit from anti-CD20 treatment may relate to inhibition of proinflammatory B cell APC function. In certain clinical settings, however, elimination of unactivated B cells, which participate in regulation of T cells and other APC, may be undesirable. Differences in immune responses to MOG protein and peptide may be important considerations when choosing an EAE model for testing novel B cell-targeting agents for MS.
Figures
Figure 1. Immunization with MOG protein, but not MOG p35–55, promotes efficient B cell APC function and development of myelin-specific antibodies
a) MACS-separated B cells (purity >95%) isolated from unimmunized (naïve) C57BL/6 mice or mice which had been immunized with CFA, MOG p35–55 or MOG protein (rMOG) were co-cultured with naïve T cells isolated from MOG T cell receptor transgenic mice in the presence of MOG p35–55 (left panel) or rMOG protein (right panel). T cell proliferation was evaluated by H3Thymidin-incorporation. C57BL/6 mice immunized with b) rMOG or c) MOG p35–55 were bled 55 days after immunization. Serum titers against rMOG (b and c, left panel) or MOG p35–55 (c, right panel) were evaluated. d) Greater numbers of B cells are detected within the CNS in EAE induced by rMOG than in EAE induced by MOG p35–55. EAE was induced in C57BL/6 mice by immunization with rMOG (100 μg) or MOG p35–55 (25 μg). CNS B cells in mice with EAE (10/group) were examined by immunohistochemistical staining for B220 on day 25 after immunization.
Figure 2. Kinetics of anti-CD20-mediated B cell depletion differs in distinct tissue microenvironments
C57BL/6 hCD20 Tg mice, were injected i.p. with 200 μg murine anti-hCD20 monoclonal antibody (m2H7) or isotype control monoclonal antibody. Cells from blood, bone marrow, lymph nodes, spleen and the peritoneal cavity were harvested at the indicated time points. Cells were stained anti-B220 (pan-B cell marker) and anti-CD21 (a mature B cell marker), then examined by FACS analysis. Results shown are representative of two experiments (2–3 mice/time-point/experiment).
Figure 3. Anti-CD20 treatment ameliorates EAE induced by mouse MOG protein
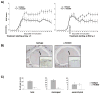
a) C57BL/6 hCD20 Tg mice received 200 μg anti-hCD20 or isotype control (IgG2a) weekly starting 21 days prior to EAE induction (left panel), or after EAE was fully established (EAE score ≥2, right panel); white arrows indicate treatment onset. EAE was scored: 0 = no clinical disease, 1 = loss of tail tone only, 2 = mild monoparesis or paraparesis, 3 = severe paraparesis, 4 = paraplegia and/or quadraparesis, and 5 = moribund or death. Results are representative of five separate experiments (10–13 mice/group/experiment). b and c) Mice receiving treatment after EAE was fully established were evaluated for the presence of B cells within spinal cord sections (B220-immunohistochemistry). Shown are b) representative spinal cord sections and c) the number of B220+ cells per square mm of total (left panel), meningeal (middle panel) or parenchymal (right panel) spinal cord tissue.
Figure 4. In EAE induced by MOG protein, anti-CD20 B cell depletion is associated with a reduced frequency of Th1-, Th17, and CD4+CD25+FoxP3+ regulatory T cells and decreased anti-MOG antibody titers
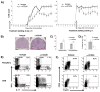
C57BL/6 hCD20 Tg mice received weekly 200 μg of anti-hCD20 or isotype (IgG2a, control) after EAE was fully established (EAE score ≥2); a) Proinflammatory differentiation of peripheral (upper panel) and CNS-infiltrating T cells (lower panel) was evaluated by intracellular FACS staining for IL-17 and IFN-γ (gated on CD3+ T cells) 14 days after onset of treatment. Frequency of peripheral (upper panel) and CNS-infiltrating FoxP3+ regulatory T cells (lower panel) was investigated by CD4/CD25/FoxP3 triple staining (gated on CD4+ T cells). b) Mice were bled weekly and evaluated for anti-MOG protein antibodies (total IgG; dilution factor 1:13,500).
Figure 5. Anti-CD20 treatment exacerbates EAE induced by MOG p35–55 peptide
a) C57BL/6 hCD20 Tg mice received 200 μg anti-hCD20 or isotype control (IgG2a) weekly starting initiated 21 days prior to EAE induction (left panel), or after EAE was fully established (EAE score ≥2, right panel); white arrows indicate treatment onset. Results are representative of four separate experiments (10–12 mice/group/experiment). b, c) Spinal cord was evaluated for inflammatory infiltration (H+E) and demyelination with sections scored on a scale from 0–4. d) Mice receiving treatment after EAE was fully established were evaluated for the presence of B cells within spinal cord sections by immunohistochemistry; shown is the number of B220+ cells per square mm of total spinal cord tissue. e) Proinflammatory differentiation of peripheral (upper panel) and CNS-infiltrating T cells (lower panel) was evaluated by intracellular FACS staining for IL-17 and IFN-γ (gated on CD3+ T cells) 14 days after treatment onset. f) Frequency of peripheral (upper panel) and CNS-infiltrating FoxP3+ regulatory T cells (lower panel) was investigated by CD4/CD25/FoxP3 triple staining (gated on CD4+ T cells).
Figure 6. Immunization with MOG protein generates a population of activated antigen-specific B cells that efficiently process and present rMOG protein to MOG 35–55 TCR Tg T cells
a) B cells isolated from C57BL/6 wild-type which had not been immunized (naïve) or immunized with CFA, MOG p35–55, OVA p323–339, MOG protein or OVA protein were evaluated for surface expression of FAS and GL7 (gated on B220+). b) MACS-separated B cells (purity >95%) isolated from unimmunized (naïve), CFA-, MOG p35–55-, or rMOG-immunized mice were co-cultured with naïve T cells isolated from MOG T cell receptor Tg mice in the presence of MOG p35–55 or rMOG protein. Proinflammatory T cell differentiation was evaluated by secretion of IFN-γ (upper panel) or IL-17 (lower panel).
Figure 7. Anti-CD20 B cell depletion increases the capacity of remaining APC to generate encephalitogenic T cells
C57BL/6 hCD20 Tg mice received weekly 0.2 mg of anti-hCD20 or isotype (IgG2a, control) starting 21 days prior to EAE induction with rMOG protein (upper panels) or MOG p35–55 peptide (lower panels). 12 days after immunization, spleens were isolated and B220+ B cells and CD3+ T cells were removed by MACS-separation. a) Production of TNF and IL-10 by remaining CD11b+ cells (gated) was evaluated by intracellular FACS staining. b) Remaining splenocytes were co-cultured with naïve T cells from MOG p35–55-specific TCR Tg mice in the presence of the antigen used for immunization. Proinflammatory T cell differentiation was evaluated by intracellular FACS staining for IL-17 and IFN-γ (gated on CD3+ T cells).
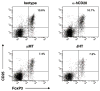
Figure 8. B cell deficiency is associated with reduced frequency of FoxP3+ regulatory T cells
Unimmunized C57BL/6 hCD20 Tg mice that received 200 μg of isotype or anti-hCD20 were compared to un-immunized C57BL/6 B cell-deficient μMT or JHT mice. Frequency of peripheral FoxP3+ regulatory T cells was investigated by CD4/CD25/FoxP3 triple staining (gated on CD4+ T cells).
Similar articles
- Anti-CD20 B-cell depletion enhances monocyte reactivity in neuroimmunological disorders.
Lehmann-Horn K, Schleich E, Hertzenberg D, Hapfelmeier A, Kümpfel T, von Bubnoff N, Hohlfeld R, Berthele A, Hemmer B, Weber MS. Lehmann-Horn K, et al. J Neuroinflammation. 2011 Oct 26;8:146. doi: 10.1186/1742-2094-8-146. J Neuroinflammation. 2011. PMID: 22027448 Free PMC article. - Treatment with MOG-DNA vaccines induces CD4+CD25+FoxP3+ regulatory T cells and up-regulates genes with neuroprotective functions in experimental autoimmune encephalomyelitis.
Fissolo N, Costa C, Nurtdinov RN, Bustamante MF, Llombart V, Mansilla MJ, Espejo C, Montalban X, Comabella M. Fissolo N, et al. J Neuroinflammation. 2012 Jun 22;9:139. doi: 10.1186/1742-2094-9-139. J Neuroinflammation. 2012. PMID: 22727044 Free PMC article. - Dynamic cross-regulation of antigen-specific effector and regulatory T cell subpopulations and microglia in brain autoimmunity.
Martinez-Pasamar S, Abad E, Moreno B, Velez de Mendizabal N, Martinez-Forero I, Garcia-Ojalvo J, Villoslada P. Martinez-Pasamar S, et al. BMC Syst Biol. 2013 Apr 26;7:34. doi: 10.1186/1752-0509-7-34. BMC Syst Biol. 2013. PMID: 23618467 Free PMC article. - Role of Th17 cells in the pathogenesis of CNS inflammatory demyelination.
Rostami A, Ciric B. Rostami A, et al. J Neurol Sci. 2013 Oct 15;333(1-2):76-87. doi: 10.1016/j.jns.2013.03.002. Epub 2013 Apr 8. J Neurol Sci. 2013. PMID: 23578791 Free PMC article. Review. - A case for regulatory B cells in controlling the severity of autoimmune-mediated inflammation in experimental autoimmune encephalomyelitis and multiple sclerosis.
Ray A, Mann MK, Basu S, Dittel BN. Ray A, et al. J Neuroimmunol. 2011 Jan;230(1-2):1-9. doi: 10.1016/j.jneuroim.2010.10.037. Epub 2010 Dec 10. J Neuroimmunol. 2011. PMID: 21145597 Free PMC article. Review.
Cited by
- MOG CNS Autoimmunity and MOGAD.
Moseley CE, Virupakshaiah A, Forsthuber TG, Steinman L, Waubant E, Zamvil SS. Moseley CE, et al. Neurol Neuroimmunol Neuroinflamm. 2024 Sep;11(5):e200275. doi: 10.1212/NXI.0000000000200275. Epub 2024 Jul 12. Neurol Neuroimmunol Neuroinflamm. 2024. PMID: 38996203 Free PMC article. Review. - The survival and function of IL-10-producing regulatory B cells are negatively controlled by SLAMF5.
Radomir L, Kramer MP, Perpinial M, Schottlender N, Rabani S, David K, Wiener A, Lewinsky H, Becker-Herman S, Aharoni R, Milo R, Mauri C, Shachar I. Radomir L, et al. Nat Commun. 2021 Mar 25;12(1):1893. doi: 10.1038/s41467-021-22230-z. Nat Commun. 2021. PMID: 33767202 Free PMC article. - Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter.
Varrin-Doyer M, Spencer CM, Schulze-Topphoff U, Nelson PA, Stroud RM, Cree BA, Zamvil SS. Varrin-Doyer M, et al. Ann Neurol. 2012 Jul;72(1):53-64. doi: 10.1002/ana.23651. Epub 2012 Jul 17. Ann Neurol. 2012. PMID: 22807325 Free PMC article. - IL-10-providing B cells govern pro-inflammatory activity of macrophages and microglia in CNS autoimmunity.
Geladaris A, Häusser-Kinzel S, Pretzsch R, Nissimov N, Lehmann-Horn K, Häusler D, Weber MS. Geladaris A, et al. Acta Neuropathol. 2023 Apr;145(4):461-477. doi: 10.1007/s00401-023-02552-6. Epub 2023 Mar 1. Acta Neuropathol. 2023. PMID: 36854993 Free PMC article. - B Cells in Neuroinflammation: New Perspectives and Mechanistic Insights.
Ahn JJ, Abu-Rub M, Miller RH. Ahn JJ, et al. Cells. 2021 Jun 26;10(7):1605. doi: 10.3390/cells10071605. Cells. 2021. PMID: 34206848 Free PMC article. Review.
References
- Prineas JW, Connell F. The fine structure of chronically active multiple sclerosis plaques. Neurology. 1978;28:68–75. - PubMed
- Genain CP, Cannella B, Hauser SL, Raine CS. Identification of autoantibodies associated with myelin damage in multiple sclerosis. Nat Med. 1999;5:170–175. - PubMed
- Keegan M, Konig F, McClelland R, et al. Relation between humoral pathological changes in multiple sclerosis and response to therapeutic plasma exchange. Lancet. 2005;366:579–582. - PubMed
- Constant S, Schweitzer N, West J, et al. B lymphocytes can be competent antigen-presenting cells for priming CD4+ T cells to protein antigens in vivo. J Immunol. 1995;155:3734–3741. - PubMed
- Constant S, Sant’Angelo D, Pasqualini T, et al. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J Immunol. 1995;154:4915–4923. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous