Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer - PubMed (original) (raw)
Inhibition of PI3K/AKT and MAPK/ERK pathways causes activation of FOXO transcription factor, leading to cell cycle arrest and apoptosis in pancreatic cancer
Sanjit K Roy et al. J Mol Signal. 2010.
Abstract
Background: Mammalian forkhead members of the class O (FOXO) transcription factors, including FOXO1, FOXO3a, and FOXO4, are implicated in the regulation of several biological processes, including the stress resistance, metabolism, cell cycle, apoptosis and DNA repair. The objectives of this study were to examine the molecular mechanisms by which FOXO transcription factors induced cell cycle arrest and apoptosis and enhanced anti-proliferative effects of sulforaphane (SFN, an active compound in cruciferous vegetables) in pancreatic cancer cells.
Results: Our data demonstrated that SFN inhibited cell proliferation and colony formation, and induced apoptosis through caspase-3 activation in pancreatic cancer cells. The inhibition of PI3K/AKT and MEK/ERK pathways activated FOXO transcription factors. SFN inhibited phosphorylation of AKT and ERK, and activated FOXO transcription factors, leading to cell cycle arrest and apoptosis. Phosphorylation deficient mutants of FOXO proteins enhanced FOXO transcriptional activity, and further enhanced SFN-induced FOXO activity and apoptosis. SFN induced the expression of p21/CIP1 and p27/KIP1, and inhibited the expression of cyclin D1.
Conclusion: These data suggest that inhibition of PI3K/AKT and ERK pathways acts together to activate FOXO transcription factor and enhances SFN-induced FOXO transcriptional activity, leading to cell cycle arrest and apoptosis.
Figures
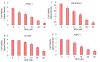
Figure 1
Effect of sulforaphane (SFN) on viability of pancreatic cancer cells. Pancreatic cancer (PANC-1, MIA PaCa-2, Hs766T and AsPC-1) cells were treated with SFN (0-30 μM) for 48 h. Cell viability was measured by XTT assay. Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05.
Figure 2
Effect of sulforaphane (SFN) on colony formation. Pancreatic cancer (PANC-1, MIA PaCa-2, Hs766T and AsPC-1) cells were treated with SFN (0-20 μM), and number of colonies were counted. Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05.
Figure 3
Effect of sulforaphane (SFN) on caspase-3 activity. Pancreatic cancer PANC-1, MIA PaCa-2, Hs 766T and AsPC-1 cells were treated with SFN (0-30 μM) for 12 h and caspase-3 activity was measured as per manufacturer's instructions (EMD Biosciences). Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05.
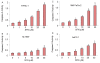
Figure 4
Effects of sulforaphane (SFN) on the expression of PTEN, AKT, and MAP kinases; and the effects of PI3K/AKT and MAPK pathways on SFN-induced apoptosis. (A), PANC-1 cells were treated with or without SFN (0-20 μM) for 24 h. The cells were harvested and the expression of PTEN, phospho-AKT, AKT, Ras, phospho-ERK, ERK, phospho-JNK, JNK, phospho-p38 and p38 was measured by Western blotting. (B), PTEN and dominant negative AKT enhance SFN-induced apoptosis. AsPC-1 and PANC-1 cells were transiently transfected with empty vector (pcDNA3.1), PTEN wild type (PTEN-WT) or dominant negative AKT (AKT-DN) along with pCMV-LacZ vector (as transfection control) for 24 h. After medium replacement, cells were treated with SFN (10 μM) for 48 h and, apoptosis was measured by Live Dead Assay. Data represent the mean ± S.D. *, # = significantly different from respective controls, P < 0.05. (C), MEK inhibitor PD98059 enhances SFN-induced apoptosis. AsPC-1 and PANC-1 cells were pretreated with PD98059 (1 μM) followed by treatment with SFN (10 μM) for 48 h and, apoptosis was measured by Live Dead Assay. Data represent the mean ± S.D. *, # = significantly different from respective controls, P < 0.05.
Figure 5
Effects of sulforaphane (SFN) on cell cycle regulatory genes. PANC-1 cells were treated with SFN (0-20 μM) for 24 h. The expression of p21/CIP1, p27/KIP1 and cyclin D1 was measured by Western blotting. Anti β-actin antibody was used as a loading control.
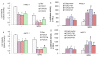
Figure 6
Effects of FOXO transcription factors on cell viability and FOXO transcriptional activity. (A and B), PANC-1 and AsPC-1 cells were transiently transfected with plasmids expressing neo (pcDNA3.1), FOXO1, FOXO3a, or FOXO4 along with pCMV-LacZ vector (as transfection control). After transfection, cells were treated with or without SFN (10 μM) for 48 h, and cell viability was measured by XTT assay. Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05. (C and D), Phosphorylation deficient mutants of FOXO enhance sulforaphane-induced FOXO transcriptional activity in pancreatic cancer. PANC-1 and AsPC-1 cells were transiently transfected with empty vector or constructs encoding FOXO1-TM, FOXO3a-TM, or FOXO4-TM together with 6X DBE-luciferase for 24 h. After transfection, cells were washed with RPMI, treated with SFN (10 μM) for 24 h, and harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control (pRL-TK; Promega). Data represent the mean ± S.D. * = significantly different from respective controls, P < 0.05.
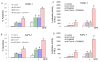
Figure 7
Inhibition of PI3K/AKT and MEK/ERK pathways synergistically/additively enhanced sulforaphane (SFN)-induced apoptosis and FOXO transcriptional activity in pancreatic cancer cells. (A and B), PANC-1 and AsPC-1 cells were pretreated with AKT inhibitor IV (1 μM) and/or MEK1/2 inhibitor PD98059 (10 μM) for 2 h, followed by treatment with SFN (10 μM) or DMSO (control) for 48 h. At the end of incubation period, cells were harvested and apoptosis was measured by TUNEL assay. Data represent mean ± SD. * = significantly different from respective controls, P < 0.05. (C and D), PANC-1 and AsPC-1 cells were transiently transfected with 6X DBE-luciferase construct for 24 h. After transfection, cells were pretreated with AKT inhibitor IV (1 μM) and/or MEK1/2 inhibitor PD98059 (10 μM) for 2 h, followed by treatment with SFN (10 μM) or DMSO (control) for 24 h. Cells were harvested for firefly/Renilla luciferase assays using the Dual-Luciferase Reporter Assay System (Promega). Luciferase counts were normalized using Renilla luciferase transfection control (pRL-TK; Promega). Data represent the mean ± S.D. *, #, ** = significantly different from respective controls, P < 0.05.
Similar articles
- Resveratrol inhibits growth of orthotopic pancreatic tumors through activation of FOXO transcription factors.
Roy SK, Chen Q, Fu J, Shankar S, Srivastava RK. Roy SK, et al. PLoS One. 2011;6(9):e25166. doi: 10.1371/journal.pone.0025166. Epub 2011 Sep 27. PLoS One. 2011. PMID: 21980390 Free PMC article. - Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells.
Chen Q, Ganapathy S, Singh KP, Shankar S, Srivastava RK. Chen Q, et al. PLoS One. 2010 Dec 14;5(12):e15288. doi: 10.1371/journal.pone.0015288. PLoS One. 2010. PMID: 21179458 Free PMC article. - Curcumin induces apoptosis in pancreatic cancer cells through the induction of forkhead box O1 and inhibition of the PI3K/Akt pathway.
Zhao Z, Li C, Xi H, Gao Y, Xu D. Zhao Z, et al. Mol Med Rep. 2015 Oct;12(4):5415-22. doi: 10.3892/mmr.2015.4060. Epub 2015 Jul 8. Mol Med Rep. 2015. PMID: 26166196 - The discrete roles of individual FOXO transcription factor family members in B-cell malignancies.
Lees J, Hay J, Moles MW, Michie AM. Lees J, et al. Front Immunol. 2023 May 18;14:1179101. doi: 10.3389/fimmu.2023.1179101. eCollection 2023. Front Immunol. 2023. PMID: 37275916 Free PMC article. Review. - FOXO transcription factors in cell-cycle regulation and the response to oxidative stress.
Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. Furukawa-Hibi Y, et al. Antioxid Redox Signal. 2005 May-Jun;7(5-6):752-60. doi: 10.1089/ars.2005.7.752. Antioxid Redox Signal. 2005. PMID: 15890021 Review.
Cited by
- A recent update on the connection between dietary phytochemicals and skin cancer: emerging understanding of the molecular mechanism.
Singh H, Mishra AK, Mohanto S, Kumar A, Mishra A, Amin R, Darwin CR, Emran TB. Singh H, et al. Ann Med Surg (Lond). 2024 Aug 7;86(10):5877-5913. doi: 10.1097/MS9.0000000000002392. eCollection 2024 Oct. Ann Med Surg (Lond). 2024. PMID: 39359831 Free PMC article. Review. - Hepatitis B virus X protein represses miRNA-148a to enhance tumorigenesis.
Xu X, Fan Z, Kang L, Han J, Jiang C, Zheng X, Zhu Z, Jiao H, Lin J, Jiang K, Ding L, Zhang H, Cheng L, Fu H, Song Y, Jiang Y, Liu J, Wang R, Du N, Ye Q. Xu X, et al. J Clin Invest. 2013 Feb;123(2):630-45. doi: 10.1172/JCI64265. Epub 2013 Jan 16. J Clin Invest. 2013. PMID: 23321675 Free PMC article. - Computational identification and modeling of crosstalk between phosphorylation, O-β-glycosylation and methylation of FoxO3 and implications for cancer therapeutics.
Butt AM, Feng D, Idrees M, Tong Y, Lu J. Butt AM, et al. Int J Mol Sci. 2012;13(3):2918-2938. doi: 10.3390/ijms13032918. Epub 2012 Mar 5. Int J Mol Sci. 2012. PMID: 22489133 Free PMC article. - Extracts from Pulsatilla patens target cancer-related signaling pathways in HeLa cells.
Łaska G, Maciejewska-Turska M, Sieniawska E, Świątek Ł, Pasco DS, Balachandran P. Łaska G, et al. Sci Rep. 2021 May 20;11(1):10654. doi: 10.1038/s41598-021-90136-3. Sci Rep. 2021. PMID: 34017038 Free PMC article. - Resveratrol inhibits bile acid-induced gastric intestinal metaplasia via the PI3K/AKT/p-FoxO4 signalling pathway.
Lu W, Ni Z, Jiang S, Tong M, Zhang J, Zhao J, Feng C, Jia Q, Wang J, Yao T, Ning H, Shi Y. Lu W, et al. Phytother Res. 2021 Mar;35(3):1495-1507. doi: 10.1002/ptr.6915. Epub 2020 Oct 25. Phytother Res. 2021. PMID: 33103284 Free PMC article.
References
- Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–465. - PubMed
- Fahey JW, Haristoy X, Dolan PM, Kensler TW, Scholtus I, Stephenson KK, Talalay P, Lozniewski A. Sulforaphane inhibits extracellular, intracellular, and antibiotic-resistant strains of Helicobacter pylori and prevents benzo[a]pyrene-induced stomach tumors. Proc Natl Acad Sci USA. 2002;99:7610–7615. doi: 10.1073/pnas.112203099. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous