Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer delays third-line chemotherapy and prolongs the platinum-free interval - PubMed (original) (raw)
Clinical Trial
doi: 10.1093/annonc/mdq353. Epub 2010 Jul 19.
N Colombo 2, B J Monk 3, S Tjulandin 4, B Kong 5, M Roy 6, S Chan 7, E Filipczyk-Cisarz 8, H Hagberg 9, I Vergote 10, C Lebedinsky 11, T Parekh 12, P Santabárbara 11, Y C Park 12, A Nieto 11, A Poveda 13
Affiliations
- PMID: 20643863
- PMCID: PMC3003617
- DOI: 10.1093/annonc/mdq353
Clinical Trial
Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer delays third-line chemotherapy and prolongs the platinum-free interval
S B Kaye et al. Ann Oncol. 2011 Jan.
Abstract
Background: OVA-301 is a large randomized trial that showed superiority of trabectedin plus pegylated liposomal doxorubicin (PLD; CentoCor Ortho Biotech Products L.P., Raritan, NJ, USA). over single-agent PLD in 672 patients with relapsed ovarian cancer, particularly in the partially platinum-sensitive subgroup [platinum-free interval (PFI) of 6-12 months]. This superiority has been suggested to be due to the differential impact of subsequent (platinum) therapy.
Patients and methods: a detailed analysis of subsequent therapies and survival outcomes in the overall population and in the subsets according to platinum sensitivity was therefore conducted.
Results: similar proportions of patients received subsequent therapy in each arm (76% versus 77%), including further platinum-based regimens (49% versus 55%). Patients in the trabectedin/PLD arm received subsequent chemotherapy at a later time (median delay 2.5 months versus PLD arm). Overall survival from subsequent platinum was significantly prolonged in the partially platinum-sensitive disease subset (hazard ratio = 0.63; P = 0.0357).
Conclusion: the superiority of trabectedin/PLD over single-agent PLD in OVA-301 cannot be explained by differences in the extent or nature of subsequent therapies administered to these patients. On the other hand, these exploratory analyses support the hypothesis that the enhanced survival benefits in the partially platinum-sensitive subset might be due to an extended PFI leading to longer survival with subsequent platinum.
Figures
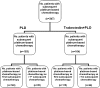
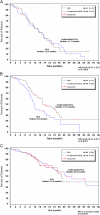
Figure 1.
Flow chart of patients receiving platinum as further chemotherapy. PLD, pegylated liposomal doxorubicin.
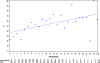
Figure 2.
Percentage of patients receiving subsequent platinum therapies per platinum-free interval. PFI, platinum-free interval (P < 0.0001).
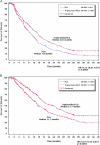
Figure 3.
Time to subsequent chemotherapy in the overall population (N = 672 patients). (A) All chemotherapy regimens. (B) Platinum-based regimens. C, number of censored patients; HR, hazard ratio; N, number of patients; P, log-rank test P value; PLD, pegylated liposomal doxorubicin.
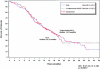
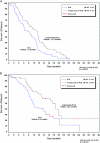
Figure 4.
Median overall survival from the administration of subsequent platinum-based therapy (all patients who received further platinum; n = 347). C, number of censored patients; HR, hazard ratio; N, number of patients; P, log-rank test P value; PLD, pegylated liposomal doxorubicin.
Figure 5.
Median overall survival from the administration of subsequent platinum-based therapy per platinum sensitivity subset (all patients who received further platinum). (A) Platinum-resistant (PFI < 6 months; _n_ = 86); (B) Partially platinum sensitive (PFI 6–12 months; _n_ = 121); (C) Platinum sensitive (PFI > 12 months; n = 139). C, number of censored patients; HR, hazard ratio; P, log-rank test P value; PLD, pegylated liposomal doxorubicin.
Figure 6.
Time to subsequent chemotherapy and median OS from the administration of platinum-based therapy as subsequent line in the platinum-free interval 6–12 subset. (A) Time to subsequent chemotherapy; (B) Overall survival. C, number of censored patients; HR, hazard ratio; P, log-rank test P value; PLD, pegylated liposomal doxorubicin; OS, overall survival.
Comment in
- Trabectedin in ovarian cancer: could we expect more?
Sessa C, D'Incalci M. Sessa C, et al. Ann Oncol. 2011 Jan;22(1):7-8. doi: 10.1093/annonc/mdq641. Epub 2010 Nov 8. Ann Oncol. 2011. PMID: 21059638 No abstract available.
Similar articles
- Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum-sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial.
Poveda A, Vergote I, Tjulandin S, Kong B, Roy M, Chan S, Filipczyk-Cisarz E, Hagberg H, Kaye SB, Colombo N, Lebedinsky C, Parekh T, Gómez J, Park YC, Alfaro V, Monk BJ. Poveda A, et al. Ann Oncol. 2011 Jan;22(1):39-48. doi: 10.1093/annonc/mdq352. Epub 2010 Jul 19. Ann Oncol. 2011. PMID: 20643862 Free PMC article. Clinical Trial. - Trabectedin plus pegylated liposomal doxorubicin in the treatment of patients with partially platinum-sensitive ovarian cancer: current evidence and future perspectives.
Sehouli J, Alfaro V, González-Martín A. Sehouli J, et al. Ann Oncol. 2012 Mar;23(3):556-562. doi: 10.1093/annonc/mdr321. Epub 2011 Jul 6. Ann Oncol. 2012. PMID: 21734221 Review. - Trabectedin plus pegylated liposomal doxorubicin (PLD) versus PLD in recurrent ovarian cancer: overall survival analysis.
Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, Pujade-Lauraine E, Park YC, Parekh TV, Poveda AM. Monk BJ, et al. Eur J Cancer. 2012 Oct;48(15):2361-8. doi: 10.1016/j.ejca.2012.04.001. Epub 2012 Apr 26. Eur J Cancer. 2012. PMID: 22541893 Clinical Trial. - Trabectedin plus pegylated liposomal Doxorubicin in recurrent ovarian cancer.
Monk BJ, Herzog TJ, Kaye SB, Krasner CN, Vermorken JB, Muggia FM, Pujade-Lauraine E, Lisyanskaya AS, Makhson AN, Rolski J, Gorbounova VA, Ghatage P, Bidzinski M, Shen K, Ngan HY, Vergote IB, Nam JH, Park YC, Lebedinsky CA, Poveda AM. Monk BJ, et al. J Clin Oncol. 2010 Jul 1;28(19):3107-14. doi: 10.1200/JCO.2009.25.4037. Epub 2010 Jun 1. J Clin Oncol. 2010. PMID: 20516432 Clinical Trial. - Optimizing treatment of the partially platinum-sensitive ovarian cancer patient.
Colombo N. Colombo N. Future Oncol. 2013 Dec;9(12 Suppl):19-23. doi: 10.2217/fon.13.206. Future Oncol. 2013. PMID: 24195526 Review.
Cited by
- Pegylated liposomal doxorubicin for relapsed epithelial ovarian cancer.
Lawrie TA, Bryant A, Cameron A, Gray E, Morrison J. Lawrie TA, et al. Cochrane Database Syst Rev. 2013 Jul 9;2013(7):CD006910. doi: 10.1002/14651858.CD006910.pub2. Cochrane Database Syst Rev. 2013. PMID: 23835762 Free PMC article. Updated. Review. - Effective treatment of a patient with stage IV ovarian cancer: A case report.
Huang Z, Yan H, Chavan D, Yuan Z, Yang X, Zhang Y, Song K, Kong B. Huang Z, et al. Oncol Lett. 2018 Jan;15(1):588-591. doi: 10.3892/ol.2017.7285. Epub 2017 Oct 30. Oncol Lett. 2018. PMID: 29285202 Free PMC article. - Trabectedin plus pegylated liposomal doxorubicin in relapsed ovarian cancer: outcomes in the partially platinum-sensitive (platinum-free interval 6-12 months) subpopulation of OVA-301 phase III randomized trial.
Poveda A, Vergote I, Tjulandin S, Kong B, Roy M, Chan S, Filipczyk-Cisarz E, Hagberg H, Kaye SB, Colombo N, Lebedinsky C, Parekh T, Gómez J, Park YC, Alfaro V, Monk BJ. Poveda A, et al. Ann Oncol. 2011 Jan;22(1):39-48. doi: 10.1093/annonc/mdq352. Epub 2010 Jul 19. Ann Oncol. 2011. PMID: 20643862 Free PMC article. Clinical Trial. - CA125-related tumor cell kinetics variables after chemotherapy in advanced ovarian cancer: a systematic review.
Colloca G, Venturino A, Governato I. Colloca G, et al. Clin Transl Oncol. 2016 Aug;18(8):813-24. doi: 10.1007/s12094-015-1441-5. Epub 2015 Nov 6. Clin Transl Oncol. 2016. PMID: 26546024 Review.
References
- Demetri GD, Chawla SP, von Mehren M, et al. Efficacy and safety of trabectedin in patients with advanced or metastatic liposarcoma or leiomyosarcoma after failure of prior anthracyclines and ifosfamide: results of a randomized phase II study of two different schedules. J Clin Oncol. 2009;27:4188–4196. - PubMed
- Carter NJ, Keam SJ. Trabectedin: a review of its use in the management of soft tissue sarcoma and ovarian cancer. Drugs. 2007;67:2257–2276. - PubMed
- Ganjoo KN, Patel SR. Trabectedin: an anticancer drug from the sea. Expert Opin Pharmacother. 2009;10:2735–2743. - PubMed
- Le Cesne A, Domont J, Cioffi A, et al. Mapping the literature: role of trabectedin as a new chemotherapy option in advanced pretreated soft tissue sarcoma. Drugs Today (Barc) 2009;45:403–421. - PubMed
- Sessa C, De Braud F, Perotti A, et al. Trabectedin for women with ovarian carcinoma after treatment with platinum and taxanes fails. J Clin Oncol. 2005;23:1867–1874. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical