Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans - PubMed (original) (raw)
Effects of oxidative stress on behavior, physiology, and the redox thiol proteome of Caenorhabditis elegans
Caroline Kumsta et al. Antioxid Redox Signal. 2011.
Abstract
Accumulation of reactive oxygen species has been implicated in various diseases and aging. However, the precise physiological effects of accumulating oxidants are still largely undefined. Here, we applied a short-term peroxide stress treatment to young Caenorhabditis elegans and measured behavioral, physiological, and cellular consequences. We discovered that exposure to peroxide stress causes a number of immediate changes, including loss in mobility, decreased growth rate, and decreased cellular adenosine triphosphate levels. Many of these alterations, which are highly reminiscent of changes in aging animals, are reversible, suggesting the presence of effective antioxidant systems in young C. elegans. One of these antioxidant systems involves the highly abundant protein peroxiredoxin 2 (PRDX-2), whose gene deletion causes phenotypes symptomatic of chronic peroxide stress and shortens lifespan. Applying the quantitative redox proteomic technique OxICAT to oxidatively stressed wild-type and prdx-2 deletion worms, we identified oxidation-sensitive cysteines in 40 different proteins, including proteins involved in mobility and feeding (e.g., MYO-2 and LET-75), protein translation and homeostasis (e.g., elongation factor 1 [EFT-1] and heat shock protein 1), and adenosine triphosphate regeneration (e.g., nucleoside diphosphate kinase). The oxidative modification of some of these redox-sensitive cysteines may contribute to the physiological and behavioral changes observed in oxidatively stressed animals.
Figures
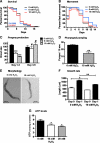
FIG. 1.
Short-term hydrogen peroxide (H2O2) treatment causes reversible behavioral defects in Caenorhabditis elegans. Synchronized wild-type C. elegans at day 0 of adulthood were treated with 0 (black trace), 6 (blue trace), or 10 m_M_ H2O2 (red trace) for 30 min in liquid M9 media. Then, the oxidant was removed and 50 worms per treatment were singled and scored for (A) survival and lifespan, (B) fast movement, (C) progeny production, (D) pharyngeal pumping (day 1), (E) morphology (day 1), and (F) growth rate (from day 0 to 1 of adulthood) at 25°C. (G) To determine intracellular adenosine triphosphate (ATP) levels, 100 μl of worms was treated with the indicated concentrations of H2O2 for 30 min, and ATP levels were measured before and after oxidative stress treatment. As seen in (A), no significant difference in the mean lifespan of oxidatively stressed worms was observed in five independent experiments. A representative life span is shown here with p = 0.3069, χ_2 = 1.044 for 6 m_M H2O2 and p = 0.6347, χ2 = 2.258 for 10 m_M_ H2O2 in comparison with the control group (0 m_M_ H2O2) (nonparametric log rank test). The movement plot shown in (B) is an average of at least three independent experiments and the direct comparison by repeated measures analysis of variance (ANOVA) and Bonferroni post hoc test reveals a difference in the movement behavior on day 0 after the oxidative stress treatment (p < 0.001). The symbols above the bars in **(C–G)** represent the _p_-values obtained using _t_-test or one-way ANOVA: #_p_ > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
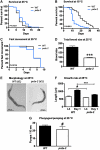
FIG. 2.
Phenotypes of prdx-2 deletion mutants. Synchronized wild-type (black trace) and prdx-2 mutant (blue trace) worms (n = 50) were singled and scored for (A, B) survival at 25°C and 15°C (C) fast movement at 25°C and (D) progeny production at 25°C. The prdx-2 mutants are significantly short-lived (p < 0.001) at 15°C **(B)** but not at 25°C (_p_ = 0.4537) **(A)**. There is no difference in the fast movement span at 25°C (_p_ = 0.9136) **(C)** and in the pharyngeal pumping rate on day 1 at 25°C (_p_ = 0.2668) **(G)**, but _prdx-2_ worms produce fewer offspring (_p_ < 0.001) **(D)**. Worms were imaged on day 0 and on day 1 after oxidative stress treatment to assess morphology (day 1) at 25°C **(E)** and growth rate from L4 stage to day 1 of adulthood **(F)**. The symbols above the bars represent the _p_-values obtained: #_p_ > 0.05, ***p < 0.001. Similar results were obtained in at least three independent experiments. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
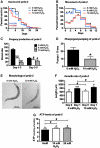
FIG. 3.
Recovery from exogenous H2O2 stress is mediated by PRDX-2. Synchronized prdx-2 C. elegans (day 0 of adulthood) were incubated with 0 (black trace), 6 (blue trace), or 10 m_M_ H2O2 (red trace) as in Figure 1. Then, the oxidant was removed and 50 worms per treatment were singled and scored for (A) survival and lifespan, (B) fast movement, (C) progeny production, (D) pharyngeal pumping (day 1), (E) morphology (day 1), and (F) growth rate (from day 0 to 1 of adulthood) at 25°C. (G) To determine intracellular ATP levels, 100 μl of worms were treated with the indicated concentrations of H2O2 for 30 min, and ATP levels were measured before and after oxidative stress treatment. As seen in (A), no significant difference in the mean lifespan of oxidatively stressed worms was observed in four independent experiments. A representative life span is shown here with p = 0.2370, χ_2 = 1.3990 for 6 m_M H2O2 and p = 0.5260, χ2 = 0.4020 for 10 m_M_ H2O2 in comparison with the control group (0 m_M_ H2O2) (nonparametric log rank test). The movement plot shown in (B) is an average of at least three independent experiments and the direct comparison by repeated measures ANOVA and Bonferroni post hoc test reveals a difference in the movement behavior on day 0 through day 3 after the oxidative stress treatment with 10 m_M_ H2O2 (p < 0.001) but not with 6 m_M_ H2O2. The symbols above the bars in **(C–G)** represent the _p_-values obtained using _t_-test or one-way ANOVA: #_p_ > 0.05, *p < 0.05, **_p_ < 0.01, ***_p_ < 0.001. The additional symbols (light gray) above the bars in **(F–G)** represent the _p_-values obtained for the comparison of _prdx-2_ worms and the respective wild-type worms presented in Figure 1F and G using two-way ANOVA and Bonferroni _post hoc_ test: #_p_ > 0.05, *p < 0.05, **p < 0.01, ***p < 0.001. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article at
).
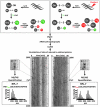
FIG. 4.
Identification of redox-sensitive C. elegans proteins using OxICAT. For the OxICAT analysis, a population of ∼100,000 worms (day 0 of adulthood) was either left untreated (left panel) or treated with 10 m_M_ H2O2 for 30 min (right panel). The worms were washed and lysed. Proteins were incubated with isotopically light 12C-ICAT reagent (green) under denaturing conditions to irreversibly label all reduced cysteines. Then, all reversibly oxidized cysteines were reduced with Tris(2-carboxyethyl)phosphine (TCEP) and subsequently irreversibly labeled with the 9 Da heavier 13C-ICAT reagent (red). The proteins were digested, and isotope coded affinity tag (ICAT)-labeled peptides were purified by affinity chromatography and analyzed using liquid chromatography (LC)/mass spectrometry (MS). MSInspect was used to illustrate the LC/MS run [for details, see Leichert et al. (30)]. The mass spectra of a typical ICAT pair harboring one oxidative stress-sensitive cysteine are shown. The mass signal with the m/z value at 2594.5 Da has incorporated one light ICAT molecule and represents the reduced form of the peptide. The mass signal with the higher m/z value of 2603.5 Da (spectra on the right) has incorporated one heavy ICAT molecule and represents the oxidized form of the peptide. MS/MS analysis revealed the identity of the protein (i.e., RPL-7) and of the redox-sensitive cysteine (i.e., Cys182). Analysis of the peak intensity revealed that oxidative stress treatment increased the oxidation status of this cysteine from 6% to 51%.
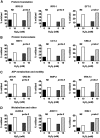
FIG. 5.
Oxidation status of select redox-sensitive C. elegans proteins. The oxidation state of cysteines in select redox-sensitive proteins involved in (A) protein translation, (B) protein homeostasis, (C) ATP metabolism and motility, and (D) metabolism and other functions is shown.
Similar articles
- Is overoxidation of peroxiredoxin physiologically significant?
Thamsen M, Kumsta C, Li F, Jakob U. Thamsen M, et al. Antioxid Redox Signal. 2011 Feb 15;14(4):725-30. doi: 10.1089/ars.2010.3717. Epub 2010 Dec 17. Antioxid Redox Signal. 2011. PMID: 20964547 Free PMC article. - A redox-sensitive peroxiredoxin that is important for longevity has tissue- and stress-specific roles in stress resistance.
Oláhová M, Taylor SR, Khazaipoul S, Wang J, Morgan BA, Matsumoto K, Blackwell TK, Veal EA. Oláhová M, et al. Proc Natl Acad Sci U S A. 2008 Dec 16;105(50):19839-44. doi: 10.1073/pnas.0805507105. Epub 2008 Dec 8. Proc Natl Acad Sci U S A. 2008. PMID: 19064914 Free PMC article. - Do developmental temperatures affect redox level and lifespan in C. elegans through upregulation of peroxiredoxin?
Henderson D, Huebner C, Markowitz M, Taube N, Harvanek ZM, Jakob U, Knoefler D. Henderson D, et al. Redox Biol. 2018 Apr;14:386-390. doi: 10.1016/j.redox.2017.10.003. Epub 2017 Oct 7. Redox Biol. 2018. PMID: 29055282 Free PMC article. - A Caenorhabditis elegans ortholog of human selenium-binding protein 1 is a pro-aging factor protecting against selenite toxicity.
Köhnlein K, Urban N, Guerrero-Gómez D, Steinbrenner H, Urbánek P, Priebs J, Koch P, Kaether C, Miranda-Vizuete A, Klotz LO. Köhnlein K, et al. Redox Biol. 2020 Jan;28:101323. doi: 10.1016/j.redox.2019.101323. Epub 2019 Sep 11. Redox Biol. 2020. PMID: 31557719 Free PMC article. - Effects of Vegetal Extracts and Metabolites against Oxidative Stress and Associated Diseases: Studies in Caenorhabditis elegans.
Hernández-Cruz EY, Eugenio-Pérez D, Ramírez-Magaña KJ, Pedraza-Chaverri J. Hernández-Cruz EY, et al. ACS Omega. 2023 Feb 27;8(10):8936-8959. doi: 10.1021/acsomega.2c07025. eCollection 2023 Mar 14. ACS Omega. 2023. PMID: 36936291 Free PMC article. Review.
Cited by
- Regulatory control or oxidative damage? Proteomic approaches to interrogate the role of cysteine oxidation status in biological processes.
Held JM, Gibson BW. Held JM, et al. Mol Cell Proteomics. 2012 Apr;11(4):R111.013037. doi: 10.1074/mcp.R111.013037. Epub 2011 Dec 8. Mol Cell Proteomics. 2012. PMID: 22159599 Free PMC article. Review. - Early perturbation in mitochondria redox homeostasis in response to environmental stress predicts cell fate in diatoms.
van Creveld SG, Rosenwasser S, Schatz D, Koren I, Vardi A. van Creveld SG, et al. ISME J. 2015 Feb;9(2):385-95. doi: 10.1038/ismej.2014.136. Epub 2014 Aug 1. ISME J. 2015. PMID: 25083933 Free PMC article. - C. elegans electrotaxis behavior is modulated by heat shock response and unfolded protein response signaling pathways.
Taylor SKB, Minhas MH, Tong J, Selvaganapathy PR, Mishra RK, Gupta BP. Taylor SKB, et al. Sci Rep. 2021 Feb 4;11(1):3115. doi: 10.1038/s41598-021-82466-z. Sci Rep. 2021. PMID: 33542359 Free PMC article. - The redoxome: Proteomic analysis of cellular redox networks.
Thamsen M, Jakob U. Thamsen M, et al. Curr Opin Chem Biol. 2011 Feb;15(1):113-9. doi: 10.1016/j.cbpa.2010.11.013. Epub 2010 Dec 2. Curr Opin Chem Biol. 2011. PMID: 21130023 Free PMC article. Review. - Interplay between redox and protein homeostasis.
Feleciano DR, Arnsburg K, Kirstein J. Feleciano DR, et al. Worm. 2016 Mar 30;5(2):e1170273. doi: 10.1080/21624054.2016.1170273. eCollection 2016 Apr-Jun. Worm. 2016. PMID: 27386166 Free PMC article. Review.
References
- Apel K. Hirt H. Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol. 2004;55:373–399. - PubMed
- Bossis G. Melchior F. Regulation of SUMOylation by reversible oxidation of SUMO conjugating enzymes. Mol Cell. 2006;21:349–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous