Symptomatic and neuroprotective effects following activation of nigral group III metabotropic glutamate receptors in rodent models of Parkinson's disease - PubMed (original) (raw)
Symptomatic and neuroprotective effects following activation of nigral group III metabotropic glutamate receptors in rodent models of Parkinson's disease
P J Austin et al. Br J Pharmacol. 2010 Aug.
Abstract
Background and purpose: Increased glutamatergic innervation of the substantia nigra pars reticulata (SNpr) and pars compacta (SNpc) may contribute to the motor deficits and neurodegeneration, respectively, in Parkinson's disease (PD). This study aimed to establish whether activation of pre-synaptic group III metabotropic glutamate (mGlu) receptors reduced glutamate release in the SN, and provided symptomatic or neuroprotective relief in animal models of PD.
Experimental approach: Broad-spectrum group III mGlu receptor agonists, O-phospho-l-serine (l-SOP) and l-2-amino-4-phosphonobutyrate (l-AP4), were assessed for their ability to inhibit KCl-evoked [(3)H]-d-aspartate release in rat nigral prisms or inhibit KCl-evoked endogenous glutamate release in the SNpr in vivo using microdialysis. Reversal of akinesia in reserpine-treated rats was assessed following intranigral injection of l-SOP and l-AP4. Finally, the neuroprotective effect of 7 days' supra-nigral treatment with l-AP4 was examined in 6-hydroxydopamine (6-OHDA)-lesioned rats.
Key results: l-SOP and l-AP4 inhibited [(3)H]-d-aspartate release by 33 and 44% respectively. These effects were blocked by the selective group III mGlu antagonist (RS)-alpha-cyclopropyl-4-phosphonophenylglycine (CPPG). l-SOP also reduced glutamate release in the SNpr in vivo by 48%. Injection of l-SOP and l-AP4 into the SNpr reversed reserpine-induced akinesia. Following administration above the SNpc, l-AP4 provided neurochemical, histological and functional protection against 6-OHDA lesion of the nigrostriatal tract. Pretreatment with CPPG inhibited these effects.
Conclusions and implications: These findings highlight group III mGlu receptors in the SN as potential targets for providing both symptomatic and neuroprotective relief in PD, and indicate that inhibition of glutamate release in the SN may underlie these effects.
Figures
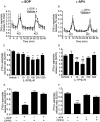
Figure 1
Effect of
l
-SOP (A,C,E) or
l
-AP4 (B,D,F) on 25 mM KCl-evoked [3H]-
d
-aspartate release in rat nigral tissue prisms. (A,B) Release profile showing effect of optimum concentration of
l
-SOP or
l
-AP4 on release evoked by the second KCl stimulus (S2). Horizontal bars indicate the periods of contact with KCl or drug/vehicle. Data are mean ± SEM (n = 3, from a single experimental run). (C,D) Graphs of S2/S1 ratio showing concentration-dependent effects of
l
-SOP or
l
-AP4 on [3H]-
d
-aspartate release. Data are mean ± SEM (n = 6); *P < 0.05 and **P < 0.01, significantly different from vehicle; ##P < 0.01 and ∞P < 0.05 significantly different from 3 and 100 µM
l
-AP4 respectively. (E,F) Effect of pre-incubation with the group III mGlu receptor antagonist, CPPG (100 µM) on responses to
l
-SOP (100 µM) or
l
-AP4 (30 µM). The presence (+) or absence (–) of drugs is indicated. Data are mean ± SEM (n = 6); ***P < 0.001 significantly different from other groups.
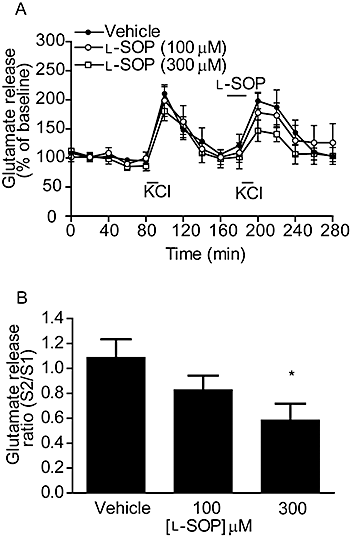
Figure 2
Effect of
l
-SOP on 100 mM KCl-evoked glutamate release in the rodent SNpr in vivo. (A) Release profile showing the effect of vehicle or
l
-SOP on release evoked by the second KCl stimulus, S2. Horizontal bars indicate the periods of contact with KCl or
l
-SOP/vehicle. (B) Graph of S2/S1 ratio showing concentration-dependent effect of
l
-SOP on glutamate release. In both cases, data are mean ± SEM (n = 4). *P < 0.05 significantly different from vehicle.
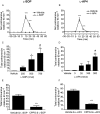
Figure 3
Effect of unilateral injection of
l
-SOP (A,C,E) or
l
-AP4 (B,D,F) in the SNpr on reversal of akinesia in the reserpine-treated rat. (A,B) Time-course of locomotor activity induced by the maximally effective dose of
l
-SOP or
l
-AP4. Data are mean ± SEM (n = 6); ***P < 0.001 significantly different from vehicle. (C,D) Effect of
l
-SOP or
l
-AP4 on rotational activity over 60 min. Data are mean ± SEM (n = 6). In (C), *P < 0.05 and **P < 0.01 significantly different from vehicle, #P < 0.05 significantly different from 250 nmol dose. In (D), ***P < 0.001 significantly different from vehicle and 3 nmol: #P < 0.05 significantly different from 30 and 100 nmol. (E,F) Effect of pretreatment with CPPG (75 nmol) on the rotational responses to
l
-SOP (750 nmol) or
l
-AP4 (300 nmol). Data are mean ± SEM (n = 6). ***P < 0.001 significantly different from vehicle pretreatment.
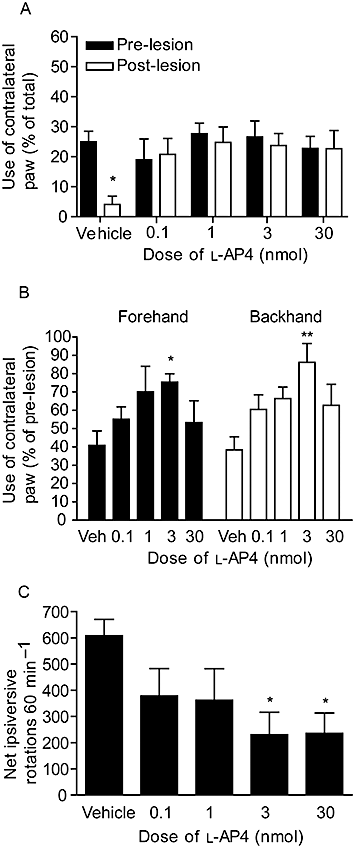
Figure 4
Effect of repeated
l
-AP4 treatment against 6-OHDA-induced (A) reduced contralateral paw use in the cylinder test at 4 days post-lesion, (B) reduced contralateral paw use in forehand or backhand adjusted stepping test at 6 days post-lesion and (C) increased amphetamine-induced ipsiversive rotations at 7 days post-lesion. Data are mean ± SEM (n = 9, vehicle; n = 6–7,
l
-AP4). *P < 0.05 and **P < 0.01, significantly different from (A) pre-lesion score or (B,C) vehicle.
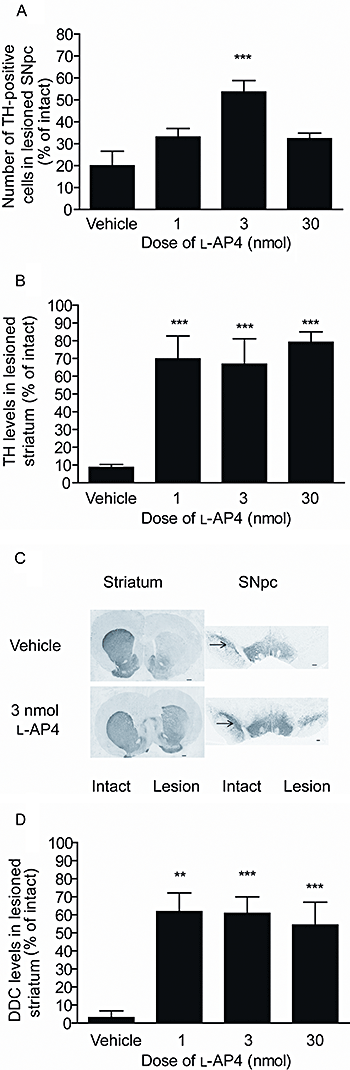
Figure 5
Effect of repeated
l
-AP4 treatment against 6-OHDA-induced (A) loss of TH-positive cells in the SNpc in the lesion hemisphere, (B) reduction in TH levels in the lesion striatum and (D) reduction in DDC levels. Data are mean ± SEM (A,B) n = 10 for vehicle; n = 6–7 for
l
-AP4; (D) n = 10 for vehicle; n = 4,7 and 8 for increasing doses of
l
-AP4. **P < 0.01 and ***P < 0.001 significantly different from vehicle (one-way
anova
with a Bonferroni post hoc test). (C) Photomicrographs showing TH staining in the striatum and SNpc of animals treated with the optimum dose of
l
-AP4 (3 nmol) or vehicle; lesion side on right. Scale bar: 500 µm for striatum; 200 µm for SNpc.
Figure 6
Effect of pretreatment with 75 nmol CPPG or vehicle on the protection afforded by 3 nmol
l
-AP4 against 6-OHDA-induced (A) reduced contralateral paw use in the cylinder test at 4 days post-lesion, (B) reduced contralateral paw use in forehand or backhand adjusted stepping test at 6 days post-lesion, (C) increased amphetamine-induced ipsiversive rotations at 7 days post-lesion and (D) reduction in striatal dopamine content. _X_-axis labels: vehicle, vehicle treatment;
l
-AP4,
l
-AP4 treatment following vehicle pretreatment; CPPG +
l
-AP4,
l
-AP4 treatment following CPPG pretreatment. Data are mean ± SEM (n = 6, vehicle; n = 7
l
-AP4 and
l
-AP4 + CPPG). (A) *P < 0.05, significantly different from pre-lesion score (two-way
anova
and Bonferroni post hoc test). (B,C,D) *P < 0.05 and ***P < 0.001 significantly different from vehicle treatment and from CPPG pretreatment followed by
l
-AP4 (one-way
anova
with Bonferroni post hoc test).
Similar articles
- Antiparkinsonian potential of targeting group III metabotropic glutamate receptor subtypes in the rodent substantia nigra pars reticulata.
Broadstock M, Austin PJ, Betts MJ, Duty S. Broadstock M, et al. Br J Pharmacol. 2012 Feb;165(4b):1034-45. doi: 10.1111/j.1476-5381.2011.01515.x. Br J Pharmacol. 2012. PMID: 21627638 Free PMC article. - Activation of group III metabotropic glutamate receptors in selected regions of the basal ganglia alleviates akinesia in the reserpine-treated rat.
MacInnes N, Messenger MJ, Duty S. MacInnes N, et al. Br J Pharmacol. 2004 Jan;141(1):15-22. doi: 10.1038/sj.bjp.0705566. Epub 2003 Nov 3. Br J Pharmacol. 2004. PMID: 14597605 Free PMC article. - Selective activation of group III metabotropic glutamate receptors by L-(+)-2-amino-4-phosphonobutryic acid protects the nigrostriatal system against 6-hydroxydopamine toxicity in vivo.
Vernon AC, Zbarsky V, Datla KP, Dexter DT, Croucher MJ. Vernon AC, et al. J Pharmacol Exp Ther. 2007 Jan;320(1):397-409. doi: 10.1124/jpet.106.108159. Epub 2006 Sep 29. J Pharmacol Exp Ther. 2007. PMID: 17012606 - Group III and subtype 4 metabotropic glutamate receptor agonists: discovery and pathophysiological applications in Parkinson's disease.
Amalric M, Lopez S, Goudet C, Fisone G, Battaglia G, Nicoletti F, Pin JP, Acher FC. Amalric M, et al. Neuropharmacology. 2013 Mar;66:53-64. doi: 10.1016/j.neuropharm.2012.05.026. Epub 2012 Jun 1. Neuropharmacology. 2013. PMID: 22664304 Review. - Therapeutic potential of targeting group III metabotropic glutamate receptors in the treatment of Parkinson's disease.
Duty S. Duty S. Br J Pharmacol. 2010 Sep;161(2):271-87. doi: 10.1111/j.1476-5381.2010.00882.x. Br J Pharmacol. 2010. PMID: 20735415 Free PMC article. Review.
Cited by
- mGlu4R, mGlu7R, and mGlu8R allosteric modulation for treating acute and chronic neurodegenerative disorders.
Domin H, Burnat G. Domin H, et al. Pharmacol Rep. 2024 Dec;76(6):1219-1241. doi: 10.1007/s43440-024-00657-7. Epub 2024 Sep 30. Pharmacol Rep. 2024. PMID: 39348087 Free PMC article. Review. - Riluzole neuroprotection in a Parkinson's disease model involves suppression of reactive astrocytosis but not GLT-1 regulation.
Carbone M, Duty S, Rattray M. Carbone M, et al. BMC Neurosci. 2012 Apr 5;13:38. doi: 10.1186/1471-2202-13-38. BMC Neurosci. 2012. PMID: 22480308 Free PMC article. - Allosteric modulation of the group III mGlu4 receptor provides functional neuroprotection in the 6-hydroxydopamine rat model of Parkinson's disease.
Betts MJ, O'Neill MJ, Duty S. Betts MJ, et al. Br J Pharmacol. 2012 Aug;166(8):2317-30. doi: 10.1111/j.1476-5381.2012.01943.x. Br J Pharmacol. 2012. PMID: 22404342 Free PMC article. - Effect of Photobiomodulation in Rescuing Lipopolysaccharide-Induced Dopaminergic Cell Loss in the Male Sprague-Dawley Rat.
O'Brien JA, Austin PJ. O'Brien JA, et al. Biomolecules. 2019 Aug 19;9(8):381. doi: 10.3390/biom9080381. Biomolecules. 2019. PMID: 31430990 Free PMC article. - Pharmacological stimulation of metabotropic glutamate receptor type 4 in a rat model of Parkinson's disease and L-DOPA-induced dyskinesia: Comparison between a positive allosteric modulator and an orthosteric agonist.
Iderberg H, Maslava N, Thompson AD, Bubser M, Niswender CM, Hopkins CR, Lindsley CW, Conn PJ, Jones CK, Cenci MA. Iderberg H, et al. Neuropharmacology. 2015 Aug;95:121-9. doi: 10.1016/j.neuropharm.2015.02.023. Epub 2015 Mar 4. Neuropharmacology. 2015. PMID: 25749357 Free PMC article.
References
- Alvarez L, Macias R, Lopez G, Alvarez E, Pavon N, Rodriguez-Oroz MC, et al. Bilateral subthalamotomy in Parkinson's disease: initial and long-term response. Brain. 2005;128:570–583. - PubMed
- Bernath S. Calcium-independent release of amino acid neurotransmitters: fact or artefact? Prog Neurobiol. 1992;38:57–91. - PubMed
- Campbell K, Kalen P, Lundberg C, Wictorin K, Rosengren E, Bjorklund A. Extracellular γ-amino butyric acid levels in the rat caudate-putamen: monitoring the neuronal and glial contribution by intracerebral microdialysis. Brain Res. 1993;614:241–250. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous