O-GlcNAc signaling in the cardiovascular system - PubMed (original) (raw)
Review
O-GlcNAc signaling in the cardiovascular system
Gladys A Ngoh et al. Circ Res. 2010.
Abstract
Cardiovascular function is regulated at multiple levels. Some of the most important aspects of such regulation involve alterations in an ever-growing list of posttranslational modifications. One such modification orchestrates input from numerous metabolic cues to modify proteins and alter their localization and/or function. Known as the beta-O-linkage of N-acetylglucosamine (ie, O-GlcNAc) to cellular proteins, this unique monosaccharide is involved in a diverse array of physiological and pathological functions. This review introduces readers to the general concepts related to O-GlcNAc, the regulation of this modification, and its role in primary pathophysiology. Much of the existing literature regarding the role of O-GlcNAcylation in disease addresses the protracted elevations in O-GlcNAcylation observed during diabetes. In this review, we focus on the emerging evidence of its involvement in the cardiovascular system. In particular, we highlight evidence of protein O-GlcNAcylation as an autoprotective alarm or stress response. We discuss recent literature supporting the idea that promoting O-GlcNAcylation improves cell survival during acute stress (eg, hypoxia, ischemia, oxidative stress), whereas limiting O-GlcNAcylation exacerbates cell damage in similar models. In addition to addressing the potential mechanisms of O-GlcNAc-mediated cardioprotection, we discuss technical issues related to studying protein O-GlcNAcylation in biological systems. The reader should gain an understanding of what protein O-GlcNAcylation is and that its roles in the acute and chronic disease settings appear distinct.
Figures
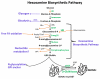
Figure 1. Hexosamine Biosynthetic Pathway
Phosphorylated glucose enters either the glycogen synthetic pathway or is further converted to fructose-6-phosphate by glucose-6-phosphate isomerase. The majority of fructose-6-phosphate is channeled to glycolysis. Less than 5% of glucose uptake is ultimately channeled to a unique accessory pathway for glucose metabolism, the hexosamine biosynthetic pathway (HBP). This pathway begins with the rate-limiting enzyme, L-glutamine:D-fructose-6-phosphate amidotransferase (GFAT), followed by acetylation of gluocsamine-6-phosphate by glucosamine-6-phosphate acetyltransferase (Emeg32) to N-acetylglucosamine-6-phosphate (GlcNAc-6-P). Next are two reversible reactions: the conversion of GlcNAc-6-P to GlcNAc-1-P by phosphate-acetylglucosamine mutase and then the formation uridine diphosphate-GlcNAc (UDP-GlcNAc) by UDP-GlcNAc pyrophosphorylase. This high-energy molecule serves as the monosaccharide donor for the post-translational modification by O-GlcNAc transferase (OGT). O-GlcNAcase (OGA) removes O-GlcNAc modification from proteins.
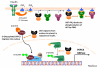
Figure 2. O-GlcNAc Affects Insulin Signaling
Insulin binds to tyrosine kinase receptor and rapidly activates intracellular signaling. PI3K gives rise to PIP3, which serves to anchor PDK1 and AKT on the membrane. According to Yang et al, PIP3 also recruits OGT to the plasma membrane where OGT attenuates insulin signaling by O-glycosylation of Thr 308, consequently inhibiting phosphorylation of Akt at the same residue. Excessive activation of OGT could disturb insulin signaling. Hyperglycemia may also stimulate the O-GlcNAcylation of CRTC2 and its migration into the nucleus, where this coactivator binds to CREB:CBP and stimulates transcription of gluconeogenic genes (PEPCK and G6Pase). The transcription factor FOXO1 is also O-GlcNAcylated under similar conditions and stimulates gluconeogenic gene transcription, .
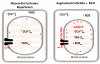
Figure 3. Mitochondria and O-GlcNAc Signaling
Myocardial ischemia induces mitochondrial Ca2+ overload and ROS generation, with subsequent mitochondrial permeability transition pore formation (mPTP). Formation of mPTP causes loss of mitochondrial trans-inner membrane potential, mitochondrial swelling, rupture of mitochondrial membrane, and cytochrome c release. Augmentation of O-GlcNAc levels prior to myocardial ischemia attenuates ischemia-induced Ca2+ overload, ROS generation, and subsequent mPTP formation. Augmented O-GlcNAc signaling also mitigates mPTP formation by possibly augmenting O-GlcNAcylation of VDAC and/or BCl-2 interaction with VDAC. This is an example of one potential mechanism; there are likely several. Moreover, augmented O-GlcNAc levels diminish mPTP-mediated mitochondrial swelling, loss of mitochondrial membrane potential, and cytochrome c release.
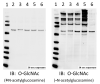
Figure 4. Common Control Measures for O-GlcNAc Immunoblots
O-GlcNAc immunoblots show multiple bands because it is a post-translational modification of numerous proteins. The blot on the left is the result of a CTD110.6 antibody co-incubated with N-acetylglucosamine, which competes for binding with the antibody. The blot on the right is the same membrane and primary antibody (CTD110.6), without GlcNAc. In addition, the lysate loaded in lane 6 is the result of a parallel aliquot of lane 5 incubated with O-GlcNAcase (in vitro), showing the loss of immunopositivity and validating the signal is O-GlcNAc. Such simple measures can confirm the fidelity of the O-GlcNAc signal via westerns.
Similar articles
- O-linked beta-N-acetylglucosamine (O-GlcNAc): Extensive crosstalk with phosphorylation to regulate signaling and transcription in response to nutrients and stress.
Butkinaree C, Park K, Hart GW. Butkinaree C, et al. Biochim Biophys Acta. 2010 Feb;1800(2):96-106. doi: 10.1016/j.bbagen.2009.07.018. Epub 2009 Aug 6. Biochim Biophys Acta. 2010. PMID: 19647786 Free PMC article. Review. - O-GlcNAcylation and oxidation of proteins: is signalling in the cardiovascular system becoming sweeter?
Lima VV, Spitler K, Choi H, Webb RC, Tostes RC. Lima VV, et al. Clin Sci (Lond). 2012 Oct;123(8):473-86. doi: 10.1042/CS20110638. Clin Sci (Lond). 2012. PMID: 22757958 Free PMC article. Review. - O-GlcNAcylation and cardiovascular disease.
Wright JN, Collins HE, Wende AR, Chatham JC. Wright JN, et al. Biochem Soc Trans. 2017 Apr 15;45(2):545-553. doi: 10.1042/BST20160164. Biochem Soc Trans. 2017. PMID: 28408494 Free PMC article. Review. - O-GlcNAc and the cardiovascular system.
Dassanayaka S, Jones SP. Dassanayaka S, et al. Pharmacol Ther. 2014 Apr;142(1):62-71. doi: 10.1016/j.pharmthera.2013.11.005. Epub 2013 Nov 25. Pharmacol Ther. 2014. PMID: 24287310 Free PMC article. Review. - Protein O-GlcNAcylation: a new signaling paradigm for the cardiovascular system.
Laczy B, Hill BG, Wang K, Paterson AJ, White CR, Xing D, Chen YF, Darley-Usmar V, Oparil S, Chatham JC. Laczy B, et al. Am J Physiol Heart Circ Physiol. 2009 Jan;296(1):H13-28. doi: 10.1152/ajpheart.01056.2008. Epub 2008 Nov 21. Am J Physiol Heart Circ Physiol. 2009. PMID: 19028792 Free PMC article.
Cited by
- Dynamic O-GlcNAcylation and its roles in the cellular stress response and homeostasis.
Groves JA, Lee A, Yildirir G, Zachara NE. Groves JA, et al. Cell Stress Chaperones. 2013 Sep;18(5):535-58. doi: 10.1007/s12192-013-0426-y. Epub 2013 Apr 26. Cell Stress Chaperones. 2013. PMID: 23620203 Free PMC article. - O-GlcNAcase overexpression reverses coronary endothelial cell dysfunction in type 1 diabetic mice.
Makino A, Dai A, Han Y, Youssef KD, Wang W, Donthamsetty R, Scott BT, Wang H, Dillmann WH. Makino A, et al. Am J Physiol Cell Physiol. 2015 Nov 1;309(9):C593-9. doi: 10.1152/ajpcell.00069.2015. Epub 2015 Aug 12. Am J Physiol Cell Physiol. 2015. PMID: 26269457 Free PMC article. - O-GlcNAcylation of 8-Oxoguanine DNA Glycosylase (Ogg1) Impairs Oxidative Mitochondrial DNA Lesion Repair in Diabetic Hearts.
Cividini F, Scott BT, Dai A, Han W, Suarez J, Diaz-Juarez J, Diemer T, Casteel DE, Dillmann WH. Cividini F, et al. J Biol Chem. 2016 Dec 16;291(51):26515-26528. doi: 10.1074/jbc.M116.754481. Epub 2016 Nov 5. J Biol Chem. 2016. PMID: 27816939 Free PMC article. - Using extracellular matrix proteomics to understand left ventricular remodeling.
Lindsey ML, Weintraub ST, Lange RA. Lindsey ML, et al. Circ Cardiovasc Genet. 2012 Feb 1;5(1):o1-7. doi: 10.1161/CIRCGENETICS.110.957803. Circ Cardiovasc Genet. 2012. PMID: 22337931 Free PMC article. Review. - With No Lysine Kinase 1 Promotes Metabolic Derangements and RV Dysfunction in Pulmonary Arterial Hypertension.
Prisco SZ, Eklund M, Raveendran R, Thenappan T, Prins KW. Prisco SZ, et al. JACC Basic Transl Sci. 2021 Nov 22;6(11):834-850. doi: 10.1016/j.jacbts.2021.09.004. eCollection 2021 Nov. JACC Basic Transl Sci. 2021. PMID: 34869947 Free PMC article.
References
- Varki A, Cummings RD, Esko JD, Freeze HH, Stanley P, Bertozzi CR, Hart GW, Etzler ME. Essentials of glycobiology. 2009:784. - PubMed
- Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266:4706–4712. - PubMed
- Paterson AJ, Kudlow JE. Regulation of glutamine:Fructose-6-phosphate amidotransferase gene transcription by epidermal growth factor and glucose. Endocrinology. 1995;136:2809–2816. - PubMed
- Chang Q, Su K, Baker JR, Yang X, Paterson AJ, Kudlow JE. Phosphorylation of human glutamine:Fructose-6-phosphate amidotransferase by camp-dependent protein kinase at serine 205 blocks the enzyme activity. J. Biol. Chem. 2000;275:21981–21987. - PubMed
- Hu Y, Riesland L, Paterson AJ, Kudlow JE. Phosphorylation of mouse glutamine-fructose-6-phosphate amidotransferase 2 (gfat2) by camp-dependent protein kinase increases the enzyme activity. J Biol Chem. 2004;279:29988–29993. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P20 RR024489/RR/NCRR NIH HHS/United States
- R01 HL083320-04/HL/NHLBI NIH HHS/United States
- R01 HL094419/HL/NHLBI NIH HHS/United States
- R01 HL083320-03/HL/NHLBI NIH HHS/United States
- R01 HL083320-03S1/HL/NHLBI NIH HHS/United States
- R01 HL094419-03/HL/NHLBI NIH HHS/United States
- R01 HL094419-02/HL/NHLBI NIH HHS/United States
- R01 HL083320-01A1/HL/NHLBI NIH HHS/United States
- R01 HL083320-05/HL/NHLBI NIH HHS/United States
- R01 HL094419-01A1/HL/NHLBI NIH HHS/United States
- P20 RR024489-026629/RR/NCRR NIH HHS/United States
- R01 HL083320/HL/NHLBI NIH HHS/United States
- R01 HL083320-02/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources