Telomerase: an RNP enzyme synthesizes DNA - PubMed (original) (raw)
Review
Telomerase: an RNP enzyme synthesizes DNA
Elizabeth H Blackburn et al. Cold Spring Harb Perspect Biol. 2011.
Abstract
Telomerase is a eukaryotic ribonucleoprotein (RNP) whose specialized reverse transcriptase action performs de novo synthesis of one strand of telomeric DNA. The resulting telomerase-mediated elongation of telomeres, which are the protective end-caps for eukaryotic chromosomes, counterbalances the inevitable attrition from incomplete DNA replication and nuclease action. The telomerase strategy to maintain telomeres is deeply conserved among eukaryotes, yet the RNA component of telomerase, which carries the built-in template for telomeric DNA repeat synthesis, has evolutionarily diverse size and sequence. Telomerase shows a distribution of labor between RNA and protein in aspects of the polymerization reaction. This article first describes the underlying conservation of a core set of structural features of telomerase RNAs important for the fundamental polymerase activity of telomerase. These include a pseudoknot-plus-template domain and at least one other RNA structural motif separate from the template-containing domain. The principles driving the diversity of telomerase RNAs are then explored. Much of the diversification of telomerase RNAs has come from apparent gain-of-function elaborations, through inferred evolutionary acquisitions of various RNA motifs used for telomerase RNP biogenesis, cellular trafficking of enzyme components, and regulation of telomerase action at telomeres. Telomerase offers broadly applicable insights into the interplay of protein and RNA functions in the context of an RNP enzyme.
Figures
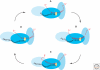
Figure 1.
The telomerase catalytic cycle. Stages in the catalytic cycle are illustrated using T. thermophila sequences of the TER template (red) and telomeric-repeat DNA (black). The active site (yellow) is within the TERT RT domain (filled with the darkest shade of blue). Some TERT-TER contacts that influence template use are stable across all states of the catalytic cycle (shown for T. thermophila as the template 5′-flanking region bound to the TERT TRBD domain in an intermediate shade of blue). Other TERT-TER and TERT-DNA contacts may be specific to particular configurations of template and product relative to the active site (shown here as changing contact between the template 3′-flanking region and the TERT TEN domain in the lightest shade of blue, which also contacts single-stranded DNA). Copying across the template with nucleotide addition processivity is accompanied by changes in the length of hybrid between template RNA and product DNA, depicted in states (A_–_C). Product released from the template can be held in association with the active enzyme by other interactions, as depicted in state (D). Realignment of the product 3′ end at the template 3′ end, as depicted in the transition from state (D) to state (A), allows for repeat addition processivity.
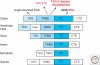
Figure 2.
Conserved TERT domains. TERT domain evolutionary conservation and protein-nucleic acid interactions are summarized, with the same color scheme of TERT and TER domains used in other figures.
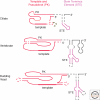
Figure 3.
Conserved TER motifs within phylogenetically divergent secondary structures. The thickest region of line represents the template, whereas dashed lines represent sites of sequence variability within the phylogenetic group. PK indicates the pseudoknot. The color scheme of TER motifs matches that in the other figures.
Similar articles
- Evolutionary perspectives of telomerase RNA structure and function.
Podlevsky JD, Chen JJ. Podlevsky JD, et al. RNA Biol. 2016 Aug 2;13(8):720-32. doi: 10.1080/15476286.2016.1205768. Epub 2016 Jun 30. RNA Biol. 2016. PMID: 27359343 Free PMC article. Review. - The RNA accordion model for template positioning by telomerase RNA during telomeric DNA synthesis.
Berman AJ, Akiyama BM, Stone MD, Cech TR. Berman AJ, et al. Nat Struct Mol Biol. 2011 Nov 20;18(12):1371-5. doi: 10.1038/nsmb.2174. Nat Struct Mol Biol. 2011. PMID: 22101935 Free PMC article. - A 4-Base-Pair Core-Enclosing Helix in Telomerase RNA Is Essential for Activity and for Binding to the Telomerase Reverse Transcriptase Catalytic Protein Subunit.
Mefford MA, Hass EP, Zappulla DC. Mefford MA, et al. Mol Cell Biol. 2020 Nov 20;40(24):e00239-20. doi: 10.1128/MCB.00239-20. Print 2020 Nov 20. Mol Cell Biol. 2020. PMID: 33046533 Free PMC article. - Human telomerase model shows the role of the TEN domain in advancing the double helix for the next polymerization step.
Steczkiewicz K, Zimmermann MT, Kurcinski M, Lewis BA, Dobbs D, Kloczkowski A, Jernigan RL, Kolinski A, Ginalski K. Steczkiewicz K, et al. Proc Natl Acad Sci U S A. 2011 Jun 7;108(23):9443-8. doi: 10.1073/pnas.1015399108. Epub 2011 May 23. Proc Natl Acad Sci U S A. 2011. PMID: 21606328 Free PMC article. - Telomerase is an unusual RNA-containing enzyme. A review.
Dokudovskaya SS, Petrov AV, Dontsova OA, Bogdanov AA. Dokudovskaya SS, et al. Biochemistry (Mosc). 1997 Nov;62(11):1206-15. Biochemistry (Mosc). 1997. PMID: 9467844 Review.
Cited by
- xRRM: a new class of RRM found in the telomerase La family protein p65.
Singh M, Choi CP, Feigon J. Singh M, et al. RNA Biol. 2013 Mar;10(3):353-9. doi: 10.4161/rna.23608. Epub 2013 Jan 17. RNA Biol. 2013. PMID: 23328630 Free PMC article. Review. - Structure of human telomerase holoenzyme with bound telomeric DNA.
Ghanim GE, Fountain AJ, van Roon AM, Rangan R, Das R, Collins K, Nguyen THD. Ghanim GE, et al. Nature. 2021 May;593(7859):449-453. doi: 10.1038/s41586-021-03415-4. Epub 2021 Apr 21. Nature. 2021. PMID: 33883742 Free PMC article. - Tetrahymena telomerase holoenzyme assembly, activation, and inhibition by domains of the p50 central hub.
Hong K, Upton H, Miracco EJ, Jiang J, Zhou ZH, Feigon J, Collins K. Hong K, et al. Mol Cell Biol. 2013 Oct;33(19):3962-71. doi: 10.1128/MCB.00792-13. Epub 2013 Aug 5. Mol Cell Biol. 2013. PMID: 23918804 Free PMC article. - Structural basis for telomerase RNA recognition and RNP assembly by the holoenzyme La family protein p65.
Singh M, Wang Z, Koo BK, Patel A, Cascio D, Collins K, Feigon J. Singh M, et al. Mol Cell. 2012 Jul 13;47(1):16-26. doi: 10.1016/j.molcel.2012.05.018. Epub 2012 Jun 14. Mol Cell. 2012. PMID: 22705372 Free PMC article. - Structure and sequence elements of the CR4/5 domain of medaka telomerase RNA important for telomerase function.
Kim NK, Zhang Q, Feigon J. Kim NK, et al. Nucleic Acids Res. 2014 Mar;42(5):3395-408. doi: 10.1093/nar/gkt1276. Epub 2013 Dec 11. Nucleic Acids Res. 2014. PMID: 24335084 Free PMC article.
References
- Autexier C, Lue NF 2006. The structure and function of telomerase reverse transcriptase. Annu Rev Biochem 75: 493–517 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous