Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex - PubMed (original) (raw)
Inhibition of the type I interferon response in human dendritic cells by dengue virus infection requires a catalytically active NS2B3 complex
Juan R Rodriguez-Madoz et al. J Virol. 2010 Oct.
Abstract
Dengue virus (DENV) is the most prevalent arthropod-borne human virus, able to infect and replicate in human dendritic cells (DCs), inducing their activation and the production of proinflammatory cytokines. However, DENV can successfully evade the immune response in order to produce disease in humans. Several mechanisms of immune evasion have been suggested for DENV, most of them involving interference with type I interferon (IFN) signaling. We recently reported that DENV infection of human DCs does not induce type I IFN production by those infected DCs, impairing their ability to prime naive T cells toward Th1 immunity. In this article, we report that DENV also reduces the ability of DCs to produce type I IFN in response to several inducers, such as infection with other viruses or exposure to Toll-like receptor (TLR) ligands, indicating that DENV antagonizes the type I IFN production pathway in human DCs. DENV-infected human DCs showed a reduced type I IFN response to Newcastle disease virus (NDV), Sendai virus (SeV), and Semliki Forest virus (SFV) infection and to the TLR3 agonist poly(I:C). This inhibitory effect is DENV dose dependent, requires DENV replication, and takes place in DENV-infected DCs as early as 2 h after infection. Expressing individual proteins of DENV in the presence of an IFN-alpha/beta production inducer reveals that a catalytically active viral protease complex is required to reduce type I IFN production significantly. These results provide a new mechanism by which DENV evades the immune system in humans.
Figures
FIG. 1.
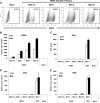
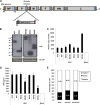
Infection of human DCs by DENV at different MOIs does not induce type I IFN production. (A) FACS analysis of the infectivities of DCs with different DENV MOIs 24 h after infection using a specific antibody against the DENV E protein. The percentage of positive cells is represented for each condition. (B to E) Quantification of the RNA levels of DENV (B), the IFN-α protein (C), IFN-α RNA (D), or IFN-β RNA (E) in DCs infected with DENV and/or NDV at the indicated MOI 24 h (white bars) or 48 h (black bars) after infection. Error bars represent standard deviations for three sample replicates from a representative donor.
FIG. 2.
Infection of DCs with DENV reduces type I IFN production after a secondary infection. DCs were infected with DENV at a MOI of 1, and 12 h later, we infected the same cells with NDV at the same MOI. Samples were collected and analyzed 18 h after secondary infection. (A) IFN-α and IFN-β RNA levels quantified by qRT-PCR. (B) IFN-α protein levels measured by specific ELISA in supernatants from infected DCs as indicated above. In this case, UV-inactivated DENV virus was included at the same MOI. (C) Analysis by Western blot of the phosphorylated and total levels of IRF-3 in DCs infected as described above. (D) Analysis of the contribution of the amount of DENV in the inhibition of type I IFN production after NDV infection. DCs were infected with DENV at the indicated MOIs, and 12 h later, DCs were infected with NDV at a MOI of 1. IFN-α protein levels were quantified in cell supernatants by ELISA 18 h after secondary infection. (E) Role that the time between infections had on the inhibition of type I IFN production. DCs were infected with DENV at a MOI of 1, and at the indicated times after DENV infection, a secondary infection with NDV was performed. Data represent the percentages of inhibition observed in IFN-α protein production after NDV infection in the group previously infected with DENV versus the mock one. (F) The ability of DENV to reduce type IFN production was not limited to a secondary infection by NDV. Twelve hours after DENV infection of DCs, a variety of IFN inducers recognized through RIG-I (NDV, SeV, and dNS1), MDA5 (SFV), or TLR3 [poly(I:C), 25 μg/ml] were used to trigger type I IFN production. Data represent the percentages of inhibition observed in IFN-α protein production after IFN triggering in the group previously infected with DENV versus the mock one. Error bars represent standard deviations for three sample replicates from a representative donor. *, P < 0.05; **, P < 0.01.
FIG. 3.
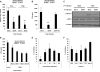
Increased NDV replication after a secondary infection of DENV-infected DCs. DCs were infected with DENV at a MOI of 1, and 12 h later, we infected the same cells with NDV at the same MOI. Mock-infected DCs or singly infected DCs with each virus were used as controls. RNA levels for DENV NS5 (A) or NDV HN (B) were measured by qRT-PCR 18 h after secondary infection. The distribution of positive cells for each virus in every group of DCs 18 h after the secondary infection was analyzed by FACS (C), and the percentage of infected DCs for each virus was quantified (D). A histogram shows GFP intensity 18 h after NDV-GFP infection of DCs previously infected with DENV (E). Max, maximum. Error bars represent standard deviations for three sample replicates from a representative donor.
FIG. 4.
Inhibition of type I IFN production by DENV after NDV infection is not a bystander effect. (A) Schematic representation of the experimental procedure in the transwell plates. Naive DCs were seeded in the lower chamber of a transwell culture plate with a membrane between the two chambers that allows the diffusion of components in the culture medium but does not allow cell-to-cell contact. Treated DCs were mock or DENV infected for 1 h and following thorough washing were placed in the upper chamber of the transwell plate. All transwell plates were cultured for 12 h, and each chamber was collected separately and subsequently mock (white bars) or NDV (black bars) infected. (B to E) Analysis of DENV NS5 RNA levels (B), NDV HN RNA levels (C), IFN-α protein levels (D), and IFN-α RNA levels (E) was done by measuring 18 h after mock or NDV infection in each chamber. Error bars represent standard deviations for three sample replicates from a representative donor. *, P < 0.05.
FIG. 5.
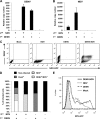
DENV NS2B3 protease complex reduces type I IFN production in human DCs. (A) Schematic representation of NDV-based vector genome with the cloning site and the insert coding for the different DENV proteins. (B) Western blot analysis of protein expression 24 h after infection of DCs with NDV vector expressing DENV proteins at a MOI of 1. (C and D) Analysis of NDV RNA (C) or IFN-α protein levels (D) 24 h after infection of DCs with NDV vectors coding for DENV proteins at a MOI of 1 is also shown. (E) Analysis of The apoptotic cells by annexin V staining in mock- or NDV-infected DCs 24 h after infection. Error bars represent standard deviations for three sample replicates from a representative donor. *, P < 0.05; **, P < 0.01. GS, gene start; GE, gene end; HA, HA tag.
FIG. 6.
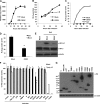
DENV infection inhibits type I IFN production in 293T cells mediated by NS2B3 protein complex. The ability of DENV to inhibit IFN-α/β production was tested in a reporter assay using 293T-IFN-β-Luc cells. (A) Replication of DENV after infection of 293T-IFN-β-Luc cells with a MOI of 1. (B and C) Luciferase levels were measured different times after infection of 293T-IFN-β-Luc cells with DENV at a MOI of 1 (B) or with SeV (C). (D) 293T-IFN-β-Luc cells were infected with DENV at a MOI of 1, and 24 h later, the same cells were infected with SeV at the same MOI. Samples were collected, and IFN-β promoter activation was measured by luciferase analysis 18 h after secondary infection. (E) Analysis by Western blotting of the phosphorylated and total levels of IRF-3 in 293T-IFN-β-Luc cells infected as described above. (F) Type I IFN production antagonist assay. 293T cells were transfected with mammalian expression plasmids coding for single DENV proteins and a reporter plasmid expressing a luciferase gene under the control of the IFN-β promoter. A plasmid expressing human TLR3 was included for TLR3-mediated IFN-β induction. IFN-β production was triggered by SeV infection or poly(I:C) (25 μg/ml) treatment, and IFN-β promoter activation was measured by determining luciferase expression. (G) Western blot analysis of the expression of the different DENV proteins 24 h after transfection of 293T cells. Error bars represent standard deviations of data from three independent experiments. *, P < 0.05; **, P < 0.01.
FIG. 7.
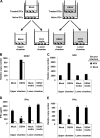
Catalytically active DENV NS2B3 protease domain is required for inhibition of type I IFN production. (A) Analysis of the effect that mutations in the catalytic triad of the DENV NS2B3 protease complex and protease domain (NS2B3pro) had on IFN-β promoter activation. 293T cells were transfected as described in Methods with mutated versions of the NS2B3 protease complex (NS2B3 S135A and H51A) or the protease domain (NS2B3pro S135A), and IFN-β production was induced by SeV infection or poly(I:C) treatment; luciferase analysis was performed as already described. wt, wild type. (B) Confirmation of impaired protease activity of mutated NS2B3 protein complex and NS2B3 protease domain. (C and D) Analysis by flow cytometry (C) or quantification (D) of apoptotic cells by annexin V staining 24 h after transfection of 293T cells with the different constructs expressing the DENV NS2B3 protease complex (thick black line). A positive control (dotted line) of induction of apoptosis an anti-CD95 (FAS) antibody and a mock-transfected group (thin line and filled plot) were included.
Similar articles
- Innate immunity evasion by Dengue virus.
Morrison J, Aguirre S, Fernandez-Sesma A. Morrison J, et al. Viruses. 2012 Mar;4(3):397-413. doi: 10.3390/v4030397. Epub 2012 Mar 15. Viruses. 2012. PMID: 22590678 Free PMC article. Review. - DENV inhibits type I IFN production in infected cells by cleaving human STING.
Aguirre S, Maestre AM, Pagni S, Patel JR, Savage T, Gutman D, Maringer K, Bernal-Rubio D, Shabman RS, Simon V, Rodriguez-Madoz JR, Mulder LC, Barber GN, Fernandez-Sesma A. Aguirre S, et al. PLoS Pathog. 2012;8(10):e1002934. doi: 10.1371/journal.ppat.1002934. Epub 2012 Oct 4. PLoS Pathog. 2012. PMID: 23055924 Free PMC article. - Dengue virus inhibits the production of type I interferon in primary human dendritic cells.
Rodriguez-Madoz JR, Bernal-Rubio D, Kaminski D, Boyd K, Fernandez-Sesma A. Rodriguez-Madoz JR, et al. J Virol. 2010 May;84(9):4845-50. doi: 10.1128/JVI.02514-09. Epub 2010 Feb 17. J Virol. 2010. PMID: 20164230 Free PMC article. - Impairment of CD4+ T cell polarization by dengue virus-infected dendritic cells.
Chase AJ, Medina FA, Muñoz-Jordán JL. Chase AJ, et al. J Infect Dis. 2011 Jun 15;203(12):1763-74. doi: 10.1093/infdis/jir197. J Infect Dis. 2011. PMID: 21606535 - How Dengue Virus Circumvents Innate Immunity.
Kao YT, Lai MMC, Yu CY. Kao YT, et al. Front Immunol. 2018 Dec 4;9:2860. doi: 10.3389/fimmu.2018.02860. eCollection 2018. Front Immunol. 2018. PMID: 30564245 Free PMC article. Review.
Cited by
- Dendritic cell apoptosis and the pathogenesis of dengue.
Martins Sde T, Silveira GF, Alves LR, Duarte dos Santos CN, Bordignon J. Martins Sde T, et al. Viruses. 2012 Nov 1;4(11):2736-53. doi: 10.3390/v4112736. Viruses. 2012. PMID: 23202502 Free PMC article. Review. - Endothelial cells elicit immune-enhancing responses to dengue virus infection.
Dalrymple NA, Mackow ER. Dalrymple NA, et al. J Virol. 2012 Jun;86(12):6408-15. doi: 10.1128/JVI.00213-12. Epub 2012 Apr 11. J Virol. 2012. PMID: 22496214 Free PMC article. - Oligomeric procyanidins stimulate innate antiviral immunity in dengue virus infected human PBMCs.
Kimmel EM, Jerome M, Holderness J, Snyder D, Kemoli S, Jutila MA, Hedges JF. Kimmel EM, et al. Antiviral Res. 2011 Apr;90(1):80-6. doi: 10.1016/j.antiviral.2011.02.011. Epub 2011 Mar 1. Antiviral Res. 2011. PMID: 21371507 Free PMC article. - Arterivirus and nairovirus ovarian tumor domain-containing Deubiquitinases target activated RIG-I to control innate immune signaling.
van Kasteren PB, Beugeling C, Ninaber DK, Frias-Staheli N, van Boheemen S, García-Sastre A, Snijder EJ, Kikkert M. van Kasteren PB, et al. J Virol. 2012 Jan;86(2):773-85. doi: 10.1128/JVI.06277-11. Epub 2011 Nov 9. J Virol. 2012. PMID: 22072774 Free PMC article. - The cGAS-STING Defense Pathway and Its Counteraction by Viruses.
Ma Z, Damania B. Ma Z, et al. Cell Host Microbe. 2016 Feb 10;19(2):150-8. doi: 10.1016/j.chom.2016.01.010. Cell Host Microbe. 2016. PMID: 26867174 Free PMC article. Review.
References
- Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124:783-801. - PubMed
- Banchereau, J., F. Briere, C. Caux, J. Davoust, S. Lebecque, Y. J. Liu, B. Pulendran, and K. Palucka. 2000. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18:767-811. - PubMed
- Basler, C. F., and A. Garcia-Sastre. 2002. Viruses and the type I interferon antiviral system: induction and evasion. Int. Rev. Immunol. 21:305-337. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources