A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model - PubMed (original) (raw)
A combination of DR5 agonistic monoclonal antibody with gemcitabine targets pancreatic cancer stem cells and results in long-term disease control in human pancreatic cancer model
N V Rajeshkumar et al. Mol Cancer Ther. 2010 Sep.
Abstract
Pancreatic ductal adenocarcinoma (PDA) is an aggressive malignancy with one of the worst outcomes among all cancers. PDA often recurs after initial treatment to result in patient death despite the use of chemotherapy or radiation therapy. PDA contains a subset of tumor-initiating cells capable of extensive self-renewal known as cancer stem cells (CSC), which may contribute to therapeutic resistance and metastasis. At present, conventional chemotherapy and radiotherapy are largely ineffective in depleting CSC pool, suggesting the need for novel therapies that specifically target the cancer-sustaining stem cells for tumor eradication and to improve the poor prognosis of PDA patients. In this study, we report that death receptor 5 (DR5) is enriched in pancreatic CSCs compared with the bulk of the tumor cells. Treating a collection of freshly generated patient-derived PDA xenografts with gemcitabine, the first-line chemotherapeutic agent for PDA, is initially effective in reducing tumor size, but largely ineffective in diminishing the CSC populations, and eventually culminated in tumor relapse. However, a combination of tigatuzumab, a fully humanized DR5 agonist monoclonal antibody, with gemcitabine proved to be more efficacious by providing a double hit to kill both CSCs and bulk tumor cells. The combination therapy produced remarkable reduction in pancreatic CSCs, tumor remissions, and significant improvements in time to tumor progression in a model that is considered more difficult to treat. These data provide the rationale to explore the DR5-directed therapies in combination with chemotherapy as a therapeutic option to improve the current standard of care for pancreatic cancer patients.
Figures
Figure 1
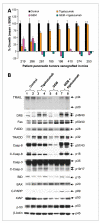
The combination of GEM and tigatuzumab produces sustained tumor growth inhibition and induces the expression of cell-extrinsic apoptotic pathway proteins in pancreatic cancer xenografts. A, in vivo efficacy of tigatuzumab, GEM, and the combination of GEM and tigatuzumab on the tumor growth of established pancreatic adenocarcinoma xenografts. Tigatuzumab monotherapy was ineffective in controlling the tumor growth of xenografts. However, the combination of GEM and tigatuzumab was highly effective in preventing the tumor growth of xenografts. Eight individual patient-derived low-passage pancreatic cancer xenografts were implanted in athymic nude mice. Cohorts of mice with a tumor volume of 200 mm3 were randomized and treated with (a) saline (vehicle); (b) tigatuzumab (3 mg/kg i.v. once a week for 4 wk); (c) GEM (100 mg/kg i.p. twice a week for 4 wk); and (d) GEM + tigatuzumab at the above-mentioned doses and frequencies. Relative tumor growth rate on day 28 of treated animals was calculated versus the tumor volume of vehicle-treated mice (100%). Cases that showed maximum sensitivity to tigatuzumab were plotted on the left side of the graph. Bars, SEM; n = 10 tumors per group (4 mice with bilateral flank tumors and 2 mice with unilateral flank tumor). B, Panc219 Western blot showing that tigatuzumab or combination with GEM induces the expression of key proteins of the extrinsic apoptotic pathway. Tumors harvested on day 28 were used for immunoblotting. Equal amounts of tumor lysates (30 μg) from two separate animals in each group were analyzed by immunoblotting and probed with the indicated antibodies. Band intensities were measured by densitometry and normalized with respective β-actin loading controls. There was an average of 2.5- and 3.0-fold increases in DR5 expression in the tigatuzumab- and GEM plus tigatuzumab–treated tumors, respectively, as compared with the saline-treated tumors. The activation of DR5 in the tigatuzumab and the combination therapy groups was coupled with the upregulation of Fas, FADD, and TRADD. Tigatuzumab and GEM plus tigatuzumab treatments lead to the upregulation of Fas (1.8- and 2.4-fold), FADD (1.5- and 2.6-fold), and TRADD (2.2- and 2.1-fold) as compared with the control tumors. GEM treatment did not modulate the expression of the above-mentioned proteins. Decreases of 1.9- and 2.4-fold in the expression of full-length and cleaved caspase-8 (C-Casp-8) were observed in the GEM-treated tumors as compared with vehicle-treated tumors. A similar downregulation of TRAIL (1.8-fold) was noticed in GEM-treated tumors as compared with vehicle-treated tumors. Both agents alone and in combination were marginally effective in reducing X-linked inhibitor of apoptosis (XIAP) expression in tumors as compared with the control tumors. There was a 2.4-fold upregulation of cleaved poly(ADP-ribose) polymerase (C-PARP), a marker of apoptosis, in the GEM plus tigatuzumab–treated tumors as compared with the GEM-treated tumors.
Figure 2
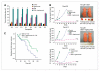
Combination therapy produces durable tumor growth inhibition and prolongs the tumor doubling time of pancreatic cancer xenografts. A, initial tumor doubling time of eight xenografts treated with tigatuzumab, GEM, and the combination of GEM and tigatuzumab. Tumors in the combination treatment group of Panc219, Panc410, Panc374, and Panc281 did not double its size as on day 120 compared with the initial tumor size. *, tumors in that group did not double its size on necropsy. There were 10 tumors each in various treatment groups until day 28 and thereafter 6 to 8 tumors in the GEM and the GEM plus tigatuzumab groups in each xenografts. Bars, SEM. B, the combination of GEM and tigatuzumab produces durable tumor growth inhibition in pancreatic cancer xenografts. Tumor growth curves representative of Panc219, Panc410, and Panc374. Number near to the asterisk denotes the number of tumors vanished during treatment. There were 10 tumors each in various treatment groups until day 28 and thereafter 6 to 8 tumors in the GEM and the GEM plus tigatuzumab groups in each xenografts. Representative images of live, anesthetized mice treated with GEM and GEM plus tigatuzumab of Panc281; excised tumors on day 120 are shown on the right. C, log-rank comparison of aggregate initial tumor doubling time of GEM-treated (n = 53) and GEM plus tigatuzumab–treated (n = 58) tumors of eight xenografts. Animals treated with the combination showed significant increase in time to tumor doubling compared with the GEM-treated mice (P = 0.002).
Figure 3
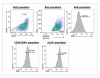
DR5 is enriched in PDA stem cells as compared with the bulk tumor cells. Tumors from mice bearing Panc219 were harvested and single-cell suspensions were generated by mincing tumors using sterile razors, followed by incubation in dispase and collagenase type IV at 37°C for 2 h with agitation. DR5+ tumor cells in the bulk tumor populations and in the ALDH+ and CD44+CD24+ tumor cell populations were measured by FACS as described in Materials and Methods. The frames represent the gates that depict respective antigen-positive tumor cells. DR5 is expressed in only 30% of bulk tumor cells. However, CSCs are relatively enriched with DR5 (94% of ALDH+ cells and 89% of CD24+CD44+ cells) as compared with the bulk tumor cells.
Figure 4
The combination of GEM and tigatuzumab markedly enhances the elimination of PDA stem cells. Panc219 tumor–bearing mice were treated with tigatuzumab, GEM, and GEM plus tigatuzumab as mentioned in Materials and Methods. The tumors were harvested on day 28 and a single-cell suspension was generated; cells positive for CSC markers were measured using flow cytometry. The frames represent the gates that depict respective antigen-positive tumor cells. Remarkable decreases in CD24+CD44+ and ALDH+ tumor cells (14.3- and 2.31-fold, respectively) were noticed in animals that received combination therapy as compared with animals treated with GEM.
Figure 5
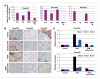
Combination therapy reduces PDA stem cells as shown by immunohistochemical staining and quantitative reverse transcription-PCR. A, CD24 staining index of Panc219, Panc410, and Panc374. Immunohistochemical staining of CD24-positive tumor cells was done as described in Materials and Methods. Index means percent of positively stained tumor cells × staining intensity (0, 1, 2, and 3). GEM treatment was not capable of reducing CD24+ tumor cells as compared with vehicle-treated tumors. Tig., tigatuzumab. B, representative micrographs of CD24 immunohistochemical staining of Panc219, Panc410, and Panc374 xenografts showing low immunoreactivity for CD24 in the tigatuzumab and the GEM and tigatuzumab combination groups as compared with vehicle-treated and GEM-treated tumors. Combination therapy leads to the complete elimination of CD24-stained tumor cells in Panc410 and Panc374 xenograft tumors. C, the combination of GEM and tigatuzumab downregulates the mRNA expression of CSC markers as compared with GEM-treated tumors. RNA isolated from Panc219 and Panc410 treatment groups was used for quantitative reverse transcription-PCR, and comparative mRNA expression of ALDH, CD44, and CD44 were calculated using the δδCT method. In Panc219, there was 6- and 3.3-fold upregulation of ALDH and CD44 mRNA in GEM-treated tumors, respectively, as compared with the control tumors. However, the combination therapy led to 4.2-, 4.0-, and 3.6-fold decreases in the expression of ALDH, CD24, and CD44 mRNA, respectively, as compared with GEM treatment (C). Similarly, mRNA expression of ALDH and CD44 were increased 8.4- and 2.3-fold, respectively, in the GEM-treated tumors of Panc410 as compared with the control tumors. Combination therapy resulted in 5.3-, 2.3-, and 3.3-fold decreases in the expression of ALDH, CD24, and CD44 mRNA, respectively, in Panc410 as compared with GEM-treated tumors (C).
Similar articles
- Aspirin counteracts cancer stem cell features, desmoplasia and gemcitabine resistance in pancreatic cancer.
Zhang Y, Liu L, Fan P, Bauer N, Gladkich J, Ryschich E, Bazhin AV, Giese NA, Strobel O, Hackert T, Hinz U, Gross W, Fortunato F, Herr I. Zhang Y, et al. Oncotarget. 2015 Apr 30;6(12):9999-10015. doi: 10.18632/oncotarget.3171. Oncotarget. 2015. PMID: 25846752 Free PMC article. - Curaxin CBL0137 eradicates drug resistant cancer stem cells and potentiates efficacy of gemcitabine in preclinical models of pancreatic cancer.
Burkhart C, Fleyshman D, Kohrn R, Commane M, Garrigan J, Kurbatov V, Toshkov I, Ramachandran R, Martello L, Gurova KV. Burkhart C, et al. Oncotarget. 2014 Nov 30;5(22):11038-53. doi: 10.18632/oncotarget.2701. Oncotarget. 2014. PMID: 25402820 Free PMC article. - Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer.
Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A, Rajeshkumar NV. Yabuuchi S, et al. Cancer Lett. 2013 Jul 10;335(1):41-51. doi: 10.1016/j.canlet.2013.01.054. Epub 2013 Feb 10. Cancer Lett. 2013. PMID: 23402814 Free PMC article. - Gemcitabine resistance in pancreatic ductal adenocarcinoma.
Binenbaum Y, Na'ara S, Gil Z. Binenbaum Y, et al. Drug Resist Updat. 2015 Nov;23:55-68. doi: 10.1016/j.drup.2015.10.002. Epub 2015 Nov 3. Drug Resist Updat. 2015. PMID: 26690340 Review. - Barriers and opportunities for gemcitabine in pancreatic cancer therapy.
Beutel AK, Halbrook CJ. Beutel AK, et al. Am J Physiol Cell Physiol. 2023 Feb 1;324(2):C540-C552. doi: 10.1152/ajpcell.00331.2022. Epub 2022 Dec 26. Am J Physiol Cell Physiol. 2023. PMID: 36571444 Free PMC article. Review.
Cited by
- Blockade of autophagy reduces pancreatic cancer stem cell activity and potentiates the tumoricidal effect of gemcitabine.
Yang MC, Wang HC, Hou YC, Tung HL, Chiu TJ, Shan YS. Yang MC, et al. Mol Cancer. 2015 Oct 12;14:179. doi: 10.1186/s12943-015-0449-3. Mol Cancer. 2015. PMID: 26458814 Free PMC article. - HuR's post-transcriptional regulation of Death Receptor 5 in pancreatic cancer cells.
Pineda DM, Rittenhouse DW, Valley CC, Cozzitorto JA, Burkhart RA, Leiby B, Winter JM, Weber MC, Londin ER, Rigoutsos I, Yeo CJ, Gorospe M, Witkiewicz AK, Sachs JN, Brody JR. Pineda DM, et al. Cancer Biol Ther. 2012 Aug;13(10):946-55. doi: 10.4161/cbt.20952. Epub 2012 Aug 1. Cancer Biol Ther. 2012. PMID: 22785201 Free PMC article. - Integrated preclinical and clinical development of S-trans, trans-Farnesylthiosalicylic Acid (FTS, Salirasib) in pancreatic cancer.
Laheru D, Shah P, Rajeshkumar NV, McAllister F, Taylor G, Goldsweig H, Le DT, Donehower R, Jimeno A, Linden S, Zhao M, Song D, Rudek MA, Hidalgo M. Laheru D, et al. Invest New Drugs. 2012 Dec;30(6):2391-9. doi: 10.1007/s10637-012-9818-6. Epub 2012 May 1. Invest New Drugs. 2012. PMID: 22547163 Free PMC article. Clinical Trial. - Personalizing cancer treatment in the age of global genomic analyses: PALB2 gene mutations and the response to DNA damaging agents in pancreatic cancer.
Villarroel MC, Rajeshkumar NV, Garrido-Laguna I, De Jesus-Acosta A, Jones S, Maitra A, Hruban RH, Eshleman JR, Klein A, Laheru D, Donehower R, Hidalgo M. Villarroel MC, et al. Mol Cancer Ther. 2011 Jan;10(1):3-8. doi: 10.1158/1535-7163.MCT-10-0893. Epub 2010 Dec 6. Mol Cancer Ther. 2011. PMID: 21135251 Free PMC article. Clinical Trial. - A missing link between RON expression and oncological outcomes in resected left-sided pancreatic cancer.
Han DH, Kang CM, Lee SW, Hwang HK, Lee WJ. Han DH, et al. Oncol Lett. 2017 Oct;14(4):4225-4230. doi: 10.3892/ol.2017.6696. Epub 2017 Aug 1. Oncol Lett. 2017. PMID: 28943931 Free PMC article.
References
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
- Ischenko I, Seeliger H, Jauch KW, Bruns CJ. Metastatic activity and chemotherapy resistance in human pancreatic cancer-influence of cancer stem cells. Surgery. 2009;146:430–4. - PubMed
- Bednar F, Simeone DM. Pancreatic cancer stem cells and relevance to cancer treatments. J Cell Biochem. 2009;107:40–5. - PubMed
- Hermann PC, Mueller MT, Heeschen C. Pancreatic cancer stem cells-insights and perspectives. Expert Opin Biol Ther. 2009;9:1271–8. - PubMed
- Li C, Heidt DG, Dalerba P, et al. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous