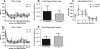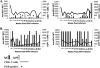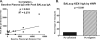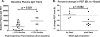Relationship of Pneumocystis jiroveci humoral immunity to prevention of colonization and chronic obstructive pulmonary disease in a primate model of HIV infection - PubMed (original) (raw)
Relationship of Pneumocystis jiroveci humoral immunity to prevention of colonization and chronic obstructive pulmonary disease in a primate model of HIV infection
Heather M Kling et al. Infect Immun. 2010 Oct.
Abstract
Pulmonary colonization by the opportunistic pathogen Pneumocystis jiroveci is common in HIV(+) subjects and has been associated with development of chronic obstructive pulmonary disease (COPD). Host and environmental factors associated with colonization susceptibility are undefined. Using a simian-human immunodeficiency virus (SHIV) model of HIV infection, the immunologic parameters associated with natural Pneumocystis jiroveci transmission were evaluated. SHIV-infected macaques were exposed to P. jiroveci by cohousing with immunosuppressed, P. jiroveci-colonized macaques in two independent experiments. Serial plasma and bronchoalveolar lavage (BAL) fluid samples were examined for changes in antibody titers to recombinant Pneumocystis-kexin protein (KEX1) and evidence of Pneumocystis colonization by nested PCR of BAL fluid. In experiment 1, 10 of 14 monkeys became Pneumocystis colonized (Pc(+)) by 8 weeks post-SHIV infection, while 4 animals remained Pneumocystis colonization negative (Pc(-)) throughout the study. In experiment 2, 11 of 17 animals became Pneumocystis colonized by 16 weeks post-SHIV infection, while 6 monkeys remained Pc(-). Baseline plasma KEX1-IgG titers were significantly higher in monkeys that remained Pc(-), compared to Pc(+) monkeys, in experiments 1 (P = 0.013) and 2 (P = 0.022). Pc(-) monkeys had greater percentages of Pneumocystis-specific memory B cells after SHIV infection compared to Pc(+) monkeys (P = 0.037). After SHIV infection, Pc(+) monkeys developed progressive obstructive pulmonary disease, whereas Pc(-) monkeys maintained normal lung function throughout the study. These results demonstrate a correlation between the KEX1 humoral response and the prevention of Pneumocystis colonization and obstructive lung disease in the SHIV model. In addition, these results indicate that an effective Pneumocystis-specific memory B-cell response is maintained despite progressive loss of CD4(+) T cells during SHIV infection.
Figures
FIG. 1.
CD4+ T-cell counts, peak plasma viral loads, and Gag antibody responses were not significantly different between Pc+ and Pc− monkeys. (A and D) Mean peripheral blood CD4+ T cells (experiment 1 [A, P = 0.488], experiment 2 [D, P = 0.326]); (B and E) peak plasma virus titers (experiment 1 [B, P = 0.749], experiment 2 [E, P = 0.595]); (C) SHIV anti-gag protein antibody titers (P = 0.419, two-way repeated measures ANOVA).
FIG. 2.
Baseline plasma anti-KEX1 IgG reciprocal endpoint titer and numbers of KEX-specific ASCs predict Pneumocystis colonization following SHIV immunosuppression. Baseline anti-KEX1 IgG titers between monkeys that became colonized after SHIV infection and monkeys that remained Pc− were analyzed. Pc− monkeys had significantly higher baseline KEX titers than monkeys that remained Pc+ in both experiment 1 (A) and experiment 2 (B). (C) Low-baseline KEX1-IgG titers are associated with Pneumocystis colonization after SHIV immunosuppression (P = 0.011, Fisher exact test). (D) Monkeys that remained Pc− had higher numbers of KEX1-specific ASCs at baseline than monkeys that became Pc+ (P = 0.018).
FIG. 3.
Representative KEX1 antibody and CD4+ T-cell profiles of macaques with low baseline KEX1 titers and high baseline KEX1 titers. Serial plasma anti-KEX1 titers and CD4 T-cell counts in two macaques with low (A) or high (B) baseline anti-KEX1 antibody titers were determined. BAL samples were tested at each time point for Pneumocystis DNA (by nested PCR). Time points marked by “+” had detectable Pneumocystis DNA.
FIG. 4.
KEX1-IgA production in the BAL fluid. Baseline plasma KEX1-IgG titers positively correlate with peak KEX1-IgA titers in the BAL fluid (A, P = 0.043). A total of 75% of Pc− macaques were producing KEX1-IgA in the BAL fluid by week 4 post-SHIV infection compared to only 10% of Pc+ macaques (B, P = 0.041, Fisher exact test).
FIG. 5.
Percentages of peripheral blood KEX1-specific memory B cells were significantly higher in Pc− monkeys than in Pc+ monkeys (experiment 2). PBMC from macaques in experimental group 2 were isolated at 9 to 12 months post-SHIV infection and stimulated in culture for 6 to 7 days and evaluated by using B-cell ELISPOT assays for total IgG and KEX1-specific IgG-secreting cells. KEX1-specific cells are expressed as a percentage of total IgG-secreting cells. The mean percentages of KEX1-specific memory cells for Pc− monkeys were significantly higher than for Pc+ monkeys (P = 0.037, Student t test).
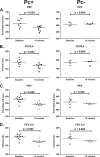
FIG. 6.
Pneumocystis colonization of immunosuppressed monkeys results in pulmonary obstruction, as measured by pulmonary function testing. Pulmonary function parameters were measured at baseline and 10 months post-SHIV infection, and changes were analyzed according to Pneumocystis colonization status. PEF (peak expiratory flow) and FEV0.4 (forced expiratory volume in 0.4 s) for Pc+ and Pc− animals in both experiment 1 (A and B) and experiment 2 (C and D) were compared by paired Student t test from baseline to 10 months post-SHIV infection. The corresponding P values for each comparison are given in each panel.
FIG. 7.
KEX1-specific antibody production is associated with protection from pulmonary function decline. (A) Monkeys that exhibited at least a 12% decline in peak expiratory flow (PEF) after immunosuppression and subsequent Pneumocystis exposure had a median baseline KEX1-IgG titer of 4,400, whereas animals that did not exhibit this decline exhibited a median baseline KEX1-IgG RET of 9,600 (P = 0.021) (combined data set from experiments 1 and 2). (B) Association of timing of KEX1-specific IgA detected in BAL fluid supernatant with PEF declines. Monkeys that produced KEX1-IgA later than 4 weeks post-SHIV infection exhibited a greater decline in PEF (mean change in PEF = 12% ± 4.3% decrease from baseline), compared to monkeys that were producing KEX1-IgA by week 4 (mean change in PEF = 1% increase) (experiment 1).
Similar articles
- Trimethoprim-sulfamethoxazole treatment does not reverse obstructive pulmonary changes in pneumocystis-colonized nonhuman primates with SHIV infection.
Kling HM, Shipley TW, Guyach S, Tarantelli R, Morris A, Norris KA. Kling HM, et al. J Acquir Immune Defic Syndr. 2014 Apr 1;65(4):381-9. doi: 10.1097/QAI.0000000000000007. J Acquir Immune Defic Syndr. 2014. PMID: 24121760 Free PMC article. - Pneumocystis colonization in immunocompetent and simian immunodeficiency virus-infected cynomolgus macaques.
Kling HM, Shipley TW, Patil S, Morris A, Norris KA. Kling HM, et al. J Infect Dis. 2009 Jan 1;199(1):89-96. doi: 10.1086/595297. J Infect Dis. 2009. PMID: 19014344 Free PMC article. - Vaccine-Induced Immunogenicity and Protection Against Pneumocystis Pneumonia in a Nonhuman Primate Model of HIV and Pneumocystis Coinfection.
Kling HM, Norris KA. Kling HM, et al. J Infect Dis. 2016 May 15;213(10):1586-95. doi: 10.1093/infdis/jiw032. Epub 2016 Jan 27. J Infect Dis. 2016. PMID: 26823337 Free PMC article. - Pneumocystis colonization, airway inflammation, and pulmonary function decline in acquired immunodeficiency syndrome.
Norris KA, Morris A, Patil S, Fernandes E. Norris KA, et al. Immunol Res. 2006;36(1-3):175-87. doi: 10.1385/IR:36:1:175. Immunol Res. 2006. PMID: 17337778 Review. - Pneumocystis jirovecii colonization in chronic pulmonary disease.
Gutiérrez S, Respaldiza N, Campano E, Martínez-Risquez MT, Calderón EJ, De La Horra C. Gutiérrez S, et al. Parasite. 2011 May;18(2):121-6. doi: 10.1051/parasite/2011182121. Parasite. 2011. PMID: 21678787 Free PMC article. Review.
Cited by
- Serologic responses to pneumocystis proteins in HIV patients with and without Pneumocystis jirovecii pneumonia.
Gingo MR, Lucht L, Daly KR, Djawe K, Palella FJ, Abraham AG, Bream JH, Witt MD, Kingsley LA, Norris KA, Walzer PD, Morris A. Gingo MR, et al. J Acquir Immune Defic Syndr. 2011 Jul 1;57(3):190-6. doi: 10.1097/QAI.0b013e3182167516. J Acquir Immune Defic Syndr. 2011. PMID: 21372726 Free PMC article. - Human immunodeficiency virus-associated obstructive lung diseases.
Gingo MR, Morris A, Crothers K. Gingo MR, et al. Clin Chest Med. 2013 Jun;34(2):273-82. doi: 10.1016/j.ccm.2013.02.002. Epub 2013 Apr 8. Clin Chest Med. 2013. PMID: 23702176 Free PMC article. Review. - Role of Pneumocystis jirovecii infection in chronic obstructive pulmonary disease progression in an immunosuppressed rat Pneumocystis pneumonia model.
Xue T, Chun-Li A. Xue T, et al. Exp Ther Med. 2020 Apr;19(4):3133-3142. doi: 10.3892/etm.2020.8545. Epub 2020 Feb 24. Exp Ther Med. 2020. PMID: 32256801 Free PMC article. - Colonization by Pneumocystis jirovecii and its role in disease.
Morris A, Norris KA. Morris A, et al. Clin Microbiol Rev. 2012 Apr;25(2):297-317. doi: 10.1128/CMR.00013-12. Clin Microbiol Rev. 2012. PMID: 22491773 Free PMC article. Review. - Pulmonary symptoms and diagnoses are associated with HIV in the MACS and WIHS cohorts.
Gingo MR, Balasubramani GK, Rice TB, Kingsley L, Kleerup EC, Detels R, Seaberg EC, Greenblatt RM, Holman S, Huang L, Sutton SH, Bertolet M, Morris A. Gingo MR, et al. BMC Pulm Med. 2014 Apr 30;14:75. doi: 10.1186/1471-2466-14-75. BMC Pulm Med. 2014. PMID: 24884738 Free PMC article.
References
- Bishop, L. R., and J. A. Kovacs. 2003. Quantitation of anti-Pneumocystis jiroveci antibodies in healthy persons and immunocompromised patients. J. Infect. Dis. 187:1844-1848. - PubMed
- Board, K. F., S. Patil, I. Lebedeva, S. Capuano III, A. M. Trichel, M. Murphey-Corb, P. A. Rajakumar, J. L. Flynn, C. G. Haidaris, and K. A. Norris. 2003. Experimental Pneumocystis carinii pneumonia in simian immunodeficiency virus-infected rhesus macaques. J. Infect. Dis. 187:576-588. - PubMed
- Calderon, E. J., C. Regordan, F. J. Medrano, M. Ollero, and J. M. Varela. 1996. Pneumocystis carinii infection in patients with chronic bronchial disease. Lancet 347:977. - PubMed
- Christensen, P. J., A. M. Preston, T. Ling, M. Du, W. B. Fields, J. L. Curtis, and J. M. Beck. 2008. Pneumocystis murina infection and cigarette smoke exposure interact to cause increased organism burden, development of airspace enlargement, and pulmonary inflammation in mice. Infect. Immun. 76:3481-3490. - PMC - PubMed
- Collins, R. L., D. E. Kanouse, A. L. Gifford, J. W. Senterfitt, M. A. Schuster, D. F. McCaffrey, M. F. Shapiro, and N. S. Wenger. 2001. Changes in health-promoting behavior following diagnosis with HIV: prevalence and correlates in a national probability sample. Health Psychol. 20:351-360. - PubMed
Publication types
MeSH terms
Grants and funding
- HL077914-01/HL/NHLBI NIH HHS/United States
- R01 HL077095/HL/NHLBI NIH HHS/United States
- T32 AI49820/AI/NIAID NIH HHS/United States
- HL077095-01A1/HL/NHLBI NIH HHS/United States
- T32 AI049820/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials