TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo - PubMed (original) (raw)
TCR ligand density and affinity determine peripheral induction of Foxp3 in vivo
Rachel A Gottschalk et al. J Exp Med. 2010.
Abstract
T cell receptor (TCR) ligation is required for the extrathymic differentiation of forkhead box p3(+) (Foxp3(+)) regulatory T cells. Several lines of evidence indicate that weak TCR stimulation favors induction of Foxp3 in the periphery; however, it remains to be determined how TCR ligand potency influences this process. We characterized the density and affinity of TCR ligand favorable for Foxp3 induction and found that a low dose of a strong agonist resulted in maximal induction of Foxp3 in vivo. Initial Foxp3 induction by weak agonist peptide could be enhanced by disruption of TCR-peptide major histocompatibility complex (pMHC) interactions or alteration of peptide dose. However, time course experiments revealed that Foxp3-positive cells induced by weak agonist stimulation are deleted, along with their Foxp3-negative counterparts, whereas Foxp3-positive cells induced by low doses of the strong agonist persist. Our results suggest that, together, pMHC ligand potency, density, and duration of TCR interactions define a cumulative quantity of TCR stimulation that determines initial peripheral Foxp3 induction. However, in the persistence of induced Foxp3(+) T cells, TCR ligand potency and density are noninterchangeable factors that influence the route to peripheral tolerance.
Figures
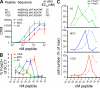
Figure 1.
There is an inverse relationship between TCR/pMHC affinity and the optimal peptide concentration for Foxp3 induction in vitro. (A) EC50 values for the experiment shown were determined as the peptide concentration resulting in 50% of maximal proliferation. LN cells from 5C.C7 TCR transgenic RAG2−/− mice were stimulated with irradiated splenocytes and the indicated peptide for 60 h. 3H-methyl-thymidine was used to assess proliferation. (B and C) 5C.C7 RAG2−/− LN cells were cultured with irradiated splenocytes in the presence of IL-2 and the indicated peptide for 4 d before flow cytometry analysis of the frequency (B) or number (C) of Foxp3+ cells. (C) Percentage of maximum cell number is shown, with Foxp3+ 5C.C7 represented as a solid line and Foxp3− cells as a dashed line. In A and B, error bars show mean ± SD, with n = 3 or n = 2 wells, respectively. Data are representative of at least three independent experiments.
Figure 2.
A single low dose of intravenous MCC peptide results in efficient peripheral induction of Foxp3. B10.A mice containing 106 naive adoptively transferred 5C.C7 RAG2−/− CD45.1 T cells were injected intravenously with the indicated dose of MCC peptide. CD4+CD45.1+ cells were assessed for expression of Foxp3 (A and B) and CD44 (in the Foxp3− population; C). (D) A time course was performed to assess Foxp3 expression and CFSE dilution of adoptively transferred 5C.C7 at each indicated day after peptide injection. The frequency of Foxp3+ cells, as a percentage of total 5C.C7, is shown on the plots. Error bars show mean ± SD of two mice per group, and data are representative of at least three independent experiments. Dot plots in A are gated on CD4+ cells, whereas all other plots and histograms are gated on CD4+CD45.1+, unless otherwise indicated.
Figure 3.
The weak agonist 102S results in a diminished frequency and number of induced Foxp3+ 5C.C7 T cells in vivo. B10.A recipients received 5C.C7 RAG2−/− CD45.1 T cells and were subsequently injected with the indicated dose of 102S, MCC, or K5 peptide. After 8 d, LN cells were assessed for the percentage (A) and number, as normalized to the endogenous CD4+CD45.2+ population (B), of 5C.C7 T cells expressing Foxp3. Error bars show mean ± SD of two mice per group and data are representative of at least three independent experiments. (C) Below the graphs are representative CD4+CD45.1+ gated dot plots, with the percentage of Foxp3+ cells shown on the plot.
Figure 4.
Disruption of TCR–pMHC interactions in vivo can inhibit proliferation while enhancing Foxp3 induction. B10.A recipients were adoptively transferred with naive CFSE-labeled CD45.1+ 5C.C7 T cells and subsequently injected with the indicated peptide followed by intravenous anti-MHCII at the indicated times. CD45.1+ LN cells were analyzed 6 d after peptide injection. (A and B) Antibody to MHCII was injected at varying time points after injection with 10 µg 102S, and the frequency of Foxp3+ 5C.C7 T cells was assessed. Representative dot plots (A) are shown beside data pooled from two independent experiments. Each point represents one mouse and horizontal bars indicate the mean (B). (C) The influence of anti-MHCII injection on the number of Foxp3+ 5C.C7 T cells was addressed by normalizing to the endogenous CD4+CD45.2+ population. n = 2 with SD. (D and E) Anti-MHCII was injected between 8 and 10 h after injection of either MCC or 102S, as indicated. 5C.C7 CFSE dilution and Foxp3 expression was assessed (D). Percentage of 5C.C7 expressing Foxp3 versus the proliferative capacity of the total 5C.C7 population are analyzed in a scatter plot. Data are pooled from at least three independent experiments, each data point representing one mouse (E). Dot plots are gated on CD4+CD45.1+ cells, and the percentages that are positive for Foxp3 are shown. All data are representative of at least three independent experiments.
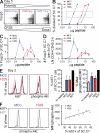
Figure 5.
In vivo, peptide dose compensates for potency to reach comparable levels of proliferation and Akt phosphorylation, but not numbers, of Foxp3-expressing 5C.C7 T cells. Naive 5C.C7 RAG2−/− CD45.1 T cells were adoptively transferred and stimulated in vivo by intravenous injection of the indicated peptide and dose. All dot plots and histograms are gated on CD4+CD45.1+ LN cells. (A) Day-5 representative dot plots for each peptide at the doses yielding comparable frequencies of divided cells and peak of Foxp3 induction: 0.3 µg MCC, 3 µg 102S, and 10 µg 102N. The percentage of divided (B), percentage of Foxp3+ (C), and number of Foxp3+ 5C.C7 T cells (D) 5 d after injection of the indicated peptide and dose are shown with SD. n = 2. The dashed rectangle in B indicates the optimal peptide doses. (E and F) 2 d after injecting doses of MCC and 102S that yield comparable CFSE dilution (0.3 µg MCC and 3 µg 102S), ki67 expression and Akt phosphorylation in total 5C.C7 T cells were measured by flow cytometry. Bar graphs in E show data pooled from three independent experiments with SD. As a control, Akt phosphorylation was compared with the maximum resulting from immunization at the base of tail with MCC and LPS, which results in a substantial portion of pAkt+ 5C.C7 T cells. Histograms in F separate CD4+CD45.1+ cells by ki67 expression, adjacent to linear regression analysis of the percentage of 5C.C7 expressing ki67 versus the phospho-Akt MFI for the total 5C.C7 population, for individual samples (best fit line shown: R2 = 0.647; P = 0.016). All data are representative of at least three independent experiments.
Figure 6.
Foxp3+ 5C.C7 induced by injection of weak agonist peptide do not persist, compared with the high-affinity ligand MCC. After adoptive transfer of 5C.C7 RAG2−/− CD45.1 T cells, recipient mice were injected with varying doses of the indicated peptides, which have been previously determined to give equivalent CFSE dilution and peak Foxp3 induction (0.3 µg MCC, 3 µg 102S, and 10 µg 102N), and then sacrificed at the indicated time points after peptide injection. (A) The percentage of Foxp3+ and numbers of Foxp3+ and Foxp3− 5C.C7 in LN were assessed at each time point. Error bars show SD of two mice per group. Representative dot plots gated on CD4+CD45.1+ cells are shown in B, with the percentage of Foxp3-positive 5C.C7 shown on the plot. Data are representative of at least three independent experiments.
Similar articles
- High TCR stimuli prevent induced regulatory T cell differentiation in a NF-κB-dependent manner.
Molinero LL, Miller ML, Evaristo C, Alegre ML. Molinero LL, et al. J Immunol. 2011 Apr 15;186(8):4609-17. doi: 10.4049/jimmunol.1002361. Epub 2011 Mar 16. J Immunol. 2011. PMID: 21411734 Free PMC article. - IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo.
Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. Chen Q, et al. J Immunol. 2011 Jun 1;186(11):6329-37. doi: 10.4049/jimmunol.1100061. Epub 2011 Apr 27. J Immunol. 2011. PMID: 21525380 Free PMC article. - A low affinity TCR ligand restores positive selection of CD8+ T cells in vivo.
Stefanski HE, Mayerova D, Jameson SC, Hogquist KA. Stefanski HE, et al. J Immunol. 2001 Jun 1;166(11):6602-7. doi: 10.4049/jimmunol.166.11.6602. J Immunol. 2001. PMID: 11359813 - Modulation of T cell function by TCR/pMHC binding kinetics.
Carreño LJ, González PA, Kalergis AM. Carreño LJ, et al. Immunobiology. 2006;211(1-2):47-64. doi: 10.1016/j.imbio.2005.09.003. Epub 2006 Jan 4. Immunobiology. 2006. PMID: 16446170 Review. - Regulatory T cells: roles of T cell receptor for their development and function.
Ohkura N, Sakaguchi S. Ohkura N, et al. Semin Immunopathol. 2010 Jun;32(2):95-106. doi: 10.1007/s00281-010-0200-5. Epub 2010 Feb 24. Semin Immunopathol. 2010. PMID: 20179931 Review.
Cited by
- Emerging patterns of regulatory T cell function in tuberculosis.
Ahmed A, Vyakarnam A. Ahmed A, et al. Clin Exp Immunol. 2020 Dec;202(3):273-287. doi: 10.1111/cei.13488. Epub 2020 Sep 6. Clin Exp Immunol. 2020. PMID: 32639588 Free PMC article. Review. - Restoring Regulatory T Cells in Type 1 Diabetes.
Spence A, Tang Q. Spence A, et al. Curr Diab Rep. 2016 Nov;16(11):110. doi: 10.1007/s11892-016-0807-6. Curr Diab Rep. 2016. PMID: 27664043 Review. - A broad range of self-reactivity drives thymic regulatory T cell selection to limit responses to self.
Lee HM, Bautista JL, Scott-Browne J, Mohan JF, Hsieh CS. Lee HM, et al. Immunity. 2012 Sep 21;37(3):475-86. doi: 10.1016/j.immuni.2012.07.009. Epub 2012 Aug 23. Immunity. 2012. PMID: 22921379 Free PMC article. - Peripheral and thymic foxp3(+) regulatory T cells in search of origin, distinction, and function.
Dhamne C, Chung Y, Alousi AM, Cooper LJ, Tran DQ. Dhamne C, et al. Front Immunol. 2013 Aug 27;4:253. doi: 10.3389/fimmu.2013.00253. eCollection 2013. Front Immunol. 2013. PMID: 23986762 Free PMC article. - Contribution of TCR signaling strength to CD8+ T cell peripheral tolerance mechanisms.
Smith TR, Verdeil G, Marquardt K, Sherman LA. Smith TR, et al. J Immunol. 2014 Oct 1;193(7):3409-16. doi: 10.4049/jimmunol.1401194. Epub 2014 Aug 25. J Immunol. 2014. PMID: 25156361 Free PMC article.
References
- Apostolou I., Sarukhan A., Klein L., von Boehmer H. 2002. Origin of regulatory T cells with known specificity for antigen. Nat. Immunol. 3:756–763 - PubMed