Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors - PubMed (original) (raw)
Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors
Jan-Philip Schülke et al. PLoS One. 2010.
Abstract
Background: Tetratricopeptide repeat (TPR) motif containing co-chaperones of the chaperone Hsp90 are considered control modules that govern activity and specificity of this central folding platform. Steroid receptors are paradigm clients of Hsp90. The influence of some TPR proteins on selected receptors has been described, but a comprehensive analysis of the effects of TPR proteins on all steroid receptors has not been accomplished yet.
Methodology and principal findings: We compared the influence of the TPR proteins FK506 binding proteins 51 and 52, protein phosphatase-5, C-terminus of Hsp70 interacting protein, cyclophillin 40, hepatitis-virus-B X-associated protein-2, and tetratricopeptide repeat protein-2 on all six steroid hormone receptors in a homogeneous mammalian cell system. To be able to assess each cofactor's effect on the transcriptional activity of on each steroid receptor we employed transient transfection in a reporter gene assay. In addition, we evaluated the interactions of the TPR proteins with the receptors and components of the Hsp90 chaperone heterocomplex by coimmunoprecipitation. In the functional assays, corticosteroid and progesterone receptors displayed the most sensitive and distinct reaction to the TPR proteins. Androgen receptor's activity was moderately impaired by most cofactors, whereas the Estrogen receptors' activity was impaired by most cofactors only to a minor degree. Second, interaction studies revealed that the strongly receptor-interacting co-chaperones were all among the inhibitory proteins. Intriguingly, the TPR-proteins also differentially co-precipitated the heterochaperone complex components Hsp90, Hsp70, and p23, pointing to differences in their modes of action.
Conclusion and significance: The results of this comprehensive study provide important insight into chaperoning of diverse client proteins via the combinatorial action of (co)-chaperones. The differential effects of the TPR proteins on steroid receptors bear on all physiological processes related to steroid hormone activity.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interests exist.
Figures
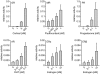
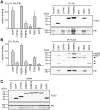
Figure 1. Response of steroid hormone receptors in MMTV-reporter gene assays.
Neuronal SK-N-MC cells were transfected with a plasmid expressing one of the HA-tagged SRs, the MMTV firefly-luciferase reporter plasmid when transfecting GR, MR, PR, or AR, an ERE firefly-luciferase reporter plasmid for ERα and ERβ, and the Gaussia-KDEL control plasmid. After transfection, the cells were cultivated for 24 h in the presence of the indicated concentrations of hormone (DHT: Dihydrotestosterone) or EtOH as solvent control. Receptor activity represents firefly data normalized to Gaussia activities + S.E.M. of at least four independent experiments, each performed in duplicate.
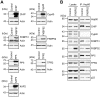
Figure 2. TPR proteins are significantly enhanced upon ectopic expression and change Hsp90 heterocomplex composition.
A, SK-N-MC cells were transfected with plasmid expressing one of the TPR proteins, lysed after 48 h and levels of the respective TPR protein was determined by Western blot analysis. B, HEK-293 cells were transfected with FLAG tagged Hsp90 along with FKBP52 expressing plasmid or control plasmid. Hsp90 was precipitated from lysates and the levels of co-precipitated cofactors were determined by Western blot.
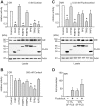
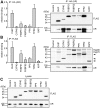
Figure 3. GR and MR activities in the presence of different TPR-proteins.
A-C, SK-N-MC cells in 96 well plates were transfected with the MMTV-Luc, Gaussia-KDEL control plasmid, a plasmid expressing one of the HA-tagged steroid hormone receptor (GR in A and B, MR in C and D) and constant amounts (200 ng) of a plasmid expressing one of the FLAG-tagged TPR-proteins. After transfection, the cells were cultivated for 24 h in the presence of hormone or vehicle as indicated. Relative receptor activity represents firefly data normalized to Gaussia activities and presented as relative stimulation to control + S.E.M. of at least four independent experiments performed in duplicate. Control cells were transfected with cloning plasmid instead of the TPR protein expressing plasmid. Lower panels of A and C, immunoblot of cell extracts, probed with anti-HA antibody visualizing steroid receptor expression, the same membrane probed with FLAG antibody demonstrating expression of TPR proteins and with actin antibody as loading control. D, After transfection, cells were cultivated in 0.1% or 10% SF-FCS containing media for 24 h in the presence of 0.03 nM fludrocortisol, or EtOH as vehicle control. Firefly luciferase data were normalized to Gaussia luciferase activities and are presented as relative stimulation + S.E.M. of three independent experiments performed in triplicate. * denotes _p-_values ≤0.001.
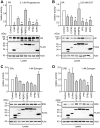
Figure 4. PR, AR, ERα and ERβ activities in the presence of different TPR proteins.
SK-N-MC cells were transfected with the MMTV-Luc (for PR and AR assays), or the ERE-Luc reporter plasmid (for ERα and ERβ assays), the Gaussia-KDEL control plasmid, a plasmid expressing the HA-tagged steroid hormone receptor as indicated and the plasmid expressing a FLAG-tagged TPR-protein. After transfection, cells were cultivated for 24 h in the presence of hormone as indicated. Relative receptor activity represents firefly data normalized to Gaussia activities and presented as relative stimulation to control + S.E.M. of at least four independent experiments performed in duplicate. Control cells were transfected with cloning plasmid replacing the TPR protein expression plasmid in the transfection mixture. Lower panels of A–D display immunoblots of cell extracts, probed with anti-HA antibody visualizing steroid receptor expression, the same membrane probed with FLAG antibody demonstrating expression of the TPR proteins and with actin antibody as loading control. * denotes _p_-values ≤0.001.
Figure 5. Estrogen receptors display little sensitivity to the Hsp90 inhibitor geldanamycin.
SK-N-MC cells were transfected with 0.25 µg of one of the plasmids expressing ERα (A), ERβ (B) or GR (C), together with either ERE-Luc (A,B) or MMTV-Luc (C) as reporter plasmid and the Gaussia-KDEL control plasmid. After transfection, the cells were cultivated for 24 h in the presence of hormone and 10 ng/ml GA as indicated. Relative receptor activity represents Firefly data normalized to Gaussia activities and is presented as relative stimulation to control + S.E.M. of at least four independent experiments performed in duplicate. Lower panels, analysis of receptor expression after GA treatment in the presence or absence of hormone (10 nM estrogen, 500 nM cortisol).
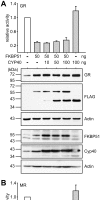
Figure 6. Cyp 40 is unable to compete the inhibitory effect of FKBP51.
SK-N-MC cells were transfected with the MMTV-Luc reporter plasmid, the Gaussia-KDEL control plasmid, one of the plasmids expressing the HA-tagged GR or MR as indicated, and plasmids expressing FKBP51 and Cyp40 at the indicated amounts. After transfection, the cells were cultivated for 24 h in the presence of 10 nM cortisol (A) or 0.03 nM Fludrocortisol (B). Bar graphs indicate the relative reporter activity representing Firefly measurements normalized to Gaussia activities and presented as relative stimulation + S.E.M. of three independent experiments performed in triplicate. Lower panel of A displays immunoblots of cell extracts, probed with HA antibody demonstrating GR expression and the same membrane probed with FLAG antibody to detect overexpressed FKBP51 and Cyp40, and actin as control. In addition, antibodies directed against FKBP51 or Cyp40 were used to visualize the combined levels of endogenous and ectopic TPR protein.
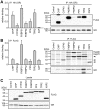
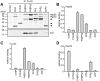
Figure 7. TPR-proteins differently interact with GR heterocomplexes.
HEK-293 cells were transfected with 5 µg of a plasmid expressing HA-tagged GR together with 2-10 µg (to achieve similar expression levels) of one of the plasmids expressing a FLAG-tagged TPR protein. After 48-72 h cultivation in SF-FCS containing media, cells were harvested, lysed, and protein extracts prepared for immunoprecipitation of either the HA-tagged GR (A), or the FLAG-tagged TPR-proteins (B). A, Precipitation of HA-GR. Displayed is an example of an immunoblot that was probed with FLAG antibody to visualize co-precipitated TPR-proteins (upper right panel), and an immunoblot of the same membrane probed with HA antibody demonstrating precipitated GR (lower right panel). Left panel, quantification of the relative binding of the TPR-proteins to the steroid receptor heterocomplexes. FLAG- and HA-immunoblot signals of the eluates and FLAG immunoblot signals of the cell extracts, demonstrating expression of TPR proteins (C), were scanned and subjected to densitometry. The signal from the co-precipitated FLAG protein was corrected first by the amount of precipitated receptor and second by the amount of the TPR-protein present in the respective cell extract. Binding of TPR-proteins is presented relative to the mean of the normalized FLAG-eluate signals of CHIP, FKBP51, FKBP52, and PP5. Quantification represents the means of three independent experiments +S.E.M. B, precipitation of TPR proteins. Upper right panel, coomassie stained gel of eluates visualizing precipitated TPR-proteins (arrowheads) and co-precipitated Hsp90 and Hsp70. Lower right panel, immunoblots of eluates probed with HA antibody to demonstrate binding of GR to TPR-protein heterocomplexes. Left panel, quantification of the relative binding of co-precipitated proteins to the precipitated TPR-proteins. For quantification, signals were scanned and subjected to densitometry. Each HA immunoblot signal of the eluate was corrected by the amount of precipitated TPR-protein. Binding of steroid receptors is presented relative to the mean of the corrected HA eluate signals. Quantifications represent means of three independent experiments +S.E.M.
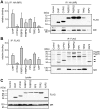
Figure 8. Differential interaction of TPR-proteins with MR heterocomplexes.
HEK-293 cells were transfected as described for figure 7, except that HA-MR was expressed instead of HA-GR. Cells were processed and protein interactions were analyzed also as described for figure 7. In A, binding of TPR-proteins is presented relative to the mean of the normalized FLAG-eluate signals of CHIP, FKBP51, FKBP52 and PP5. Quantification represents means of three independent experiments (two for TPR2) +S.E.M.. In B, binding is normalized as in figure 7. C, FLAG- and HA-immunoblot signals of the cell extracts, demonstrating expression of TPR proteins and MR. Quantifications represent means of three independent experiments +S.E.M.
Figure 9. Differential interaction of TPR-proteins with PR heterocomplexes.
HEK-293 cells were transfected as described for figure 7, except that HA-MR was expressed instead of HA-GR. Cells were processed and protein interactions were analyzed also as described for figure 7. In A, binding of TPR-proteins is presented relative to the mean of the normalized FLAG-eluate signals of CHIP, FKBP52, PP5 and TPR2. Quantification represents means of three independent experiments (two for FKBP51) +S.E.M. In B, binding is normalized as in figure 7. C, FLAG- and HA-immunoblot signals of the cell extracts, demonstrating expression of TPR proteins and PR. Quantifications represent means of three independent experiments +S.E.M.
Figure 10. Differential interaction of TPR-proteins with AR heterocomplexes.
HEK-293 cells were transfected as described for figure 7, except that HA-MR was expressed instead of HA-GR. Cells were processed and protein interactions were analyzed also as described for figure 7. In A, binding of TPR-proteins is presented relative to the mean of the normalized FLAG-eluate signals of CHIP, FKBP51, and TPR2. Quantification represents means of three independent experiments (two for XAP2) +S.E.M. In B, binding is normalized as in figure 7. C, FLAG- and HA-immunoblot signals of the cell extracts, demonstrating expression of TPR proteins and AR. Quantifications represent means of three independent experiments +S.E.M.
Figure 11. TPR cofactors differentially recruit components of the multichaperone heterocomplex.
HEK cells were transfected and TPR cofactors immunoprecipitated as described in the legends to figures 7– 10. The relative amounts of the precipitated TPR cofactors, the co-precipitated Hsp70 and Hsp90 were determined by densitometry of a coomassie stained gel of the eluates (A, upper panel), and the relative amount of p23 by densitometry of the immunoblot signals (A, lower panel). B and D, quantification of the relative binding of co-precipitated Hsp90 (B) and Hsp70 (D). Hsp90 signals and Hsp70 signals (only intensities above background binding were taken into consideration) were normalized to signals of the respective precipitated TPR cofactors. Data are presented as relative binding + S.E.M. of at least 12 independent experiments with different steroid receptors. C, quantification of the relative binding of co-precipitated p23. The p23 immunoblot signals were related (normalized) to the respective TPR cofactor signal. Binding of p23 is presented relative to the mean of the normalized p23 eluate signals of the complete set of TPR-proteins.
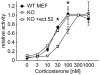
Figure 12. Loss of FKBP52 affects GR responsiveness to cortisol.
FKBP52-KO MEF cells (open symbols) or WT MEF cells (closed circles) were transfected with the MMTV-Luc reporter plasmid, the Gaussia-KDEL control plasmid, a plasmid expressing the HA-tagged mGR and either a plasmid expressing FLAG-tagged FKBP52 (+ect.52) or empty vector. After transfection, cells were cultivated for 24 h in the presence of hormone. Relative receptor activity represents firefly data normalized to Gaussia activities and is presented relative to the activity at saturating 300 nM corticosterone +S.E.M. of three independent experiments, each performed in triplicates. Significance of different receptor activation between FKBP52 KO cells and FKBP52 KO cells ectopically expressing FLAG-tagged FKBP52 was evaluated by one sampled T-test (* denotes _p_-values ≤0.001).
Similar articles
- Role of molecular chaperones and TPR-domain proteins in the cytoplasmic transport of steroid receptors and their passage through the nuclear pore.
Galigniana MD, Echeverría PC, Erlejman AG, Piwien-Pilipuk G. Galigniana MD, et al. Nucleus. 2010 Jul-Aug;1(4):299-308. doi: 10.4161/nucl.1.4.11743. Nucleus. 2010. PMID: 21113270 Free PMC article. - The cochaperone SGTA (small glutamine-rich tetratricopeptide repeat-containing protein alpha) demonstrates regulatory specificity for the androgen, glucocorticoid, and progesterone receptors.
Paul A, Garcia YA, Zierer B, Patwardhan C, Gutierrez O, Hildenbrand Z, Harris DC, Balsiger HA, Sivils JC, Johnson JL, Buchner J, Chadli A, Cox MB. Paul A, et al. J Biol Chem. 2014 May 30;289(22):15297-308. doi: 10.1074/jbc.M113.535229. Epub 2014 Apr 21. J Biol Chem. 2014. PMID: 24753260 Free PMC article. - Interaction of the Hsp90 cochaperone cyclophilin 40 with Hsc70.
Carrello A, Allan RK, Morgan SL, Owen BA, Mok D, Ward BK, Minchin RF, Toft DO, Ratajczak T. Carrello A, et al. Cell Stress Chaperones. 2004 Summer;9(2):167-81. doi: 10.1379/csc-26r.1. Cell Stress Chaperones. 2004. PMID: 15497503 Free PMC article. - Steroid receptor interactions with heat shock protein and immunophilin chaperones.
Pratt WB, Toft DO. Pratt WB, et al. Endocr Rev. 1997 Jun;18(3):306-60. doi: 10.1210/edrv.18.3.0303. Endocr Rev. 1997. PMID: 9183567 Review. - Therapeutic Targeting of the FKBP52 Co-Chaperone in Steroid Hormone Receptor-Regulated Physiology and Disease.
Guy NC, Garcia YA, Cox MB. Guy NC, et al. Curr Mol Pharmacol. 2015;9(2):109-25. doi: 10.2174/1874467208666150519114115. Curr Mol Pharmacol. 2015. PMID: 25986565 Review.
Cited by
- DnaJC7 in Amyotrophic Lateral Sclerosis.
Dilliott AA, Andary CM, Stoltz M, Petropavlovskiy AA, Farhan SMK, Duennwald ML. Dilliott AA, et al. Int J Mol Sci. 2022 Apr 7;23(8):4076. doi: 10.3390/ijms23084076. Int J Mol Sci. 2022. PMID: 35456894 Free PMC article. Review. - Downregulation of FKBP5 Promotes Atrial Arrhythmogenesis.
Wang X, Song J, Yuan Y, Li L, Abu-Taha I, Heijman J, Sun L, Dobrev S, Kamler M, Xie L, Wehrens XHT, Horrigan FT, Dobrev D, Li N. Wang X, et al. Circ Res. 2023 Jun 23;133(1):e1-e16. doi: 10.1161/CIRCRESAHA.122.322213. Epub 2023 May 8. Circ Res. 2023. PMID: 37154033 Free PMC article. - The FKBP51 Glucocorticoid Receptor Co-Chaperone: Regulation, Function, and Implications in Health and Disease.
Fries GR, Gassen NC, Rein T. Fries GR, et al. Int J Mol Sci. 2017 Dec 5;18(12):2614. doi: 10.3390/ijms18122614. Int J Mol Sci. 2017. PMID: 29206196 Free PMC article. Review. - Biological Actions of the Hsp90-binding Immunophilins FKBP51 and FKBP52.
Zgajnar NR, De Leo SA, Lotufo CM, Erlejman AG, Piwien-Pilipuk G, Galigniana MD. Zgajnar NR, et al. Biomolecules. 2019 Feb 1;9(2):52. doi: 10.3390/biom9020052. Biomolecules. 2019. PMID: 30717249 Free PMC article. Review. - DnaJC7 binds natively folded structural elements in tau to inhibit amyloid formation.
Hou Z, Wydorski PM, Perez VA, Mendoza-Oliva A, Ryder BD, Mirbaha H, Kashmer O, Joachimiak LA. Hou Z, et al. Nat Commun. 2021 Sep 9;12(1):5338. doi: 10.1038/s41467-021-25635-y. Nat Commun. 2021. PMID: 34504072 Free PMC article.
References
- Katzenellenbogen JA, O'Malley BW, Katzenellenbogen BS. Tripartite steroid hormone receptor pharmacology: interaction with multiple effector sites as a basis for the cell- and promoter-specific action of these hormones. Mol Endocrinol. 1996;10:119–131. - PubMed
- Beato M, Klug J. Steroid hormone receptors: an update. Hum Reprod Update. 2000;6:225–236. - PubMed
- Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search of a plot. Cell. 1995;83:851–857. - PubMed
- Karin M. New twists in gene regulation by glucocorticoid receptor: is DNA binding dispensable? 178. Cell. 1998;93:487–490. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous