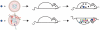Quasispecies theory and the behavior of RNA viruses - PubMed (original) (raw)
Review
Quasispecies theory and the behavior of RNA viruses
Adam S Lauring et al. PLoS Pathog. 2010.
Abstract
A large number of medically important viruses, including HIV, hepatitis C virus, and influenza, have RNA genomes. These viruses replicate with extremely high mutation rates and exhibit significant genetic diversity. This diversity allows a viral population to rapidly adapt to dynamic environments and evolve resistance to vaccines and antiviral drugs. For the last 30 years, quasispecies theory has provided a population-based framework for understanding RNA viral evolution. A quasispecies is a cloud of diverse variants that are genetically linked through mutation, interact cooperatively on a functional level, and collectively contribute to the characteristics of the population. Many predictions of quasispecies theory run counter to traditional views of microbial behavior and evolution and have profound implications for our understanding of viral disease. Here, we discuss basic principles of quasispecies theory and describe its relevance for our understanding of viral fitness, virulence, and antiviral therapeutic strategy.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
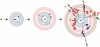
Figure 1. RNA viruses exist as a quasispecies.
A virus replicating with a high mutation rate will generate a diverse mutant repertoire over the course of a few generations. In these trees, each branch indicates two variants linked by a point mutation and the concentric circles represent serial replication cycles. The resulting distribution is often represented as a cloud centered on a master sequence. This two dimensional schematic is a vast oversimplification of the intraquasispecies connectivity. In the mathematical formulations of quasispecies theory, sequence space is multidimensional, with numerous branches between variants.
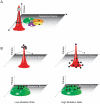
Figure 2. The fitness landscape and survival of the flattest.
(A) Population 1 has high fitness but is trapped in sequence space because mutation leads to a dramatic loss of fitness. Population 2 is more mutationally robust because mutation leads to minor fitness losses. The flatter population is ideally situated to move through sequence and access other local peaks through neighboring mutational networks (indicated in different colors). (B) At low mutation rates, variants will be genotypically stable and cluster at the top of the fitness peak. The variant with the highest fitness will easily outcompete all others. At high mutation rates, variants spread out over the corresponding peaks. Variants on the flatter peak (green) remain near their fitness optimum and have a higher mean fitness than the population located on the steeper peak (red). The flatter population will prevail.
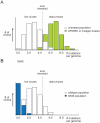
Figure 3. Mutant distributions and the error threshold.
(A) The majority of viruses in a wild-type population has few mutations and is viable Some viruses, bearing a higher mutational load, are nonviable and considered beyond the threshold of error catastrophe (shown in green). Small increases in mutation frequency, mediated by host APOBEC proteins or exogenous mutagen, push the distribution to the right, past the error threshold. The number of errors per genome is sufficiently high to lethally mutate a majority of the population. (B) A high fidelity polymerase results results in a narrower quasispecies situated farther from the error threshold. This population is more resistant to the effect of mutagen, because it does not accumulate as many mutations, as the wild type does not cross the error threshold. Figure adapted from Crotty et al. .
Figure 4. Population diversity is a virulence determinant.
Results of experiments described in Vignuzzi et al. . A neurovirulent clone of poliovirus was isolated from the brains of mice that had been infected with a wild-type strain. Naive mice were then reinfected with this clone as part of either a genetically constrained (top) or diverse population (bottom). Although all mice received the neurovirulent clone, only those infected with a diverse quasispecies developed disease. Subpopulations within the diverse quasispecies cooperated with the neurovirulent clone to facilitate its entry into the CNS.
Similar articles
- Genetic diversity in RNA virus quasispecies is controlled by host-virus interactions.
Schneider WL, Roossinck MJ. Schneider WL, et al. J Virol. 2001 Jul;75(14):6566-71. doi: 10.1128/JVI.75.14.6566-6571.2001. J Virol. 2001. PMID: 11413324 Free PMC article. - Viral quasispecies.
Domingo E, Perales C. Domingo E, et al. PLoS Genet. 2019 Oct 17;15(10):e1008271. doi: 10.1371/journal.pgen.1008271. eCollection 2019 Oct. PLoS Genet. 2019. PMID: 31622336 Free PMC article. - Viral population dynamics and virulence thresholds.
Lancaster KZ, Pfeiffer JK. Lancaster KZ, et al. Curr Opin Microbiol. 2012 Aug;15(4):525-30. doi: 10.1016/j.mib.2012.05.007. Epub 2012 Jun 2. Curr Opin Microbiol. 2012. PMID: 22658738 Free PMC article. Review. - Viral quasispecies evolution.
Domingo E, Sheldon J, Perales C. Domingo E, et al. Microbiol Mol Biol Rev. 2012 Jun;76(2):159-216. doi: 10.1128/MMBR.05023-11. Microbiol Mol Biol Rev. 2012. PMID: 22688811 Free PMC article. Review. - RNA virus quasispecies: significance for viral disease and epidemiology.
Duarte EA, Novella IS, Weaver SC, Domingo E, Wain-Hobson S, Clarke DK, Moya A, Elena SF, de la Torre JC, Holland JJ. Duarte EA, et al. Infect Agents Dis. 1994 Aug;3(4):201-14. Infect Agents Dis. 1994. PMID: 7827789 Review.
Cited by
- In vivo evidence for ribavirin-induced mutagenesis of the hepatitis E virus genome.
Todt D, Gisa A, Radonic A, Nitsche A, Behrendt P, Suneetha PV, Pischke S, Bremer B, Brown RJ, Manns MP, Cornberg M, Bock CT, Steinmann E, Wedemeyer H. Todt D, et al. Gut. 2016 Oct;65(10):1733-43. doi: 10.1136/gutjnl-2015-311000. Epub 2016 May 24. Gut. 2016. PMID: 27222534 Free PMC article. - Hepatitis E Virus Mutations: Functional and Clinical Relevance.
van Tong H, Hoan NX, Wang B, Wedemeyer H, Bock CT, Velavan TP. van Tong H, et al. EBioMedicine. 2016 Sep;11:31-42. doi: 10.1016/j.ebiom.2016.07.039. Epub 2016 Aug 6. EBioMedicine. 2016. PMID: 27528267 Free PMC article. Review. - DEVELOPMENTALLY REGULATED PLASMA MEMBRANE PROTEIN of Nicotiana benthamiana contributes to potyvirus movement and transports to plasmodesmata via the early secretory pathway and the actomyosin system.
Geng C, Cong QQ, Li XD, Mou AL, Gao R, Liu JL, Tian YP. Geng C, et al. Plant Physiol. 2015 Feb;167(2):394-410. doi: 10.1104/pp.114.252734. Epub 2014 Dec 24. Plant Physiol. 2015. PMID: 25540331 Free PMC article. - Effects of political conflict-induced treatment interruptions on HIV drug resistance.
Mann M, Lurie MN, Kimaiyo S, Kantor R. Mann M, et al. AIDS Rev. 2013 Jan-Mar;15(1):15-24. AIDS Rev. 2013. PMID: 23449225 Free PMC article. Review. - Evolutionary deletions within the SARS-CoV-2 genome as signature trends for virus fitness and adaptation.
Jeronimo PMC, Aksenen CF, Duarte IO, Lins RD, Miyajima F. Jeronimo PMC, et al. J Virol. 2024 Jan 23;98(1):e0140423. doi: 10.1128/jvi.01404-23. Epub 2023 Dec 13. J Virol. 2024. PMID: 38088350 Free PMC article. Review.
References
- Duffy S, Shackelton LA, Holmes EC. Rates of evolutionary change in viruses: patterns and determinants. Nat Rev Genet. 2008;9:267–276. - PubMed
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, et al. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. - PubMed
- Dawood FS, Jain S, Finelli L, Shaw MW, Lindstrom S, et al. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N Engl J Med. 2009;360:2605–2615. - PubMed
Publication types
MeSH terms
Grants and funding
- R01 AI40085/AI/NIAID NIH HHS/United States
- K08 AI081754-03/AI/NIAID NIH HHS/United States
- R01 AI36178/AI/NIAID NIH HHS/United States
- R01 AI036178/AI/NIAID NIH HHS/United States
- K08 AI081754-01/AI/NIAID NIH HHS/United States
- R01 AI040085/AI/NIAID NIH HHS/United States
- K08 AI081754/AI/NIAID NIH HHS/United States
- K08 AI081754-02/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources