Weekly rituximab followed by monthly rituximab treatment for steroid-refractory chronic graft-versus-host disease: results from a prospective, multicenter, phase II study - PubMed (original) (raw)
Clinical Trial
. 2010 Nov;95(11):1935-42.
doi: 10.3324/haematol.2010.026104. Epub 2010 Jul 27.
Jong Wook Lee, Chul Won Jung, Chang Ki Min, Bin Cho, Ho Jin Shin, Joo Seop Chung, Hawk Kim, Won Sik Lee, Young Don Joo, Deok-Hwan Yang, Hoon Kook, Hyoung Jin Kang, Hyo Seop Ahn, Sung-Soo Yoon, Sang Kyun Sohn, Yoo Hong Min, Woo-Sung Min, Hee-Sook Park, Jong Ho Won
Affiliations
- PMID: 20663943
- PMCID: PMC2966917
- DOI: 10.3324/haematol.2010.026104
Clinical Trial
Weekly rituximab followed by monthly rituximab treatment for steroid-refractory chronic graft-versus-host disease: results from a prospective, multicenter, phase II study
Seok Jin Kim et al. Haematologica. 2010 Nov.
Abstract
Background: Since it was suggested that B cells play a role in the pathogenesis of chronic graft-versus-host disease, rituximab, an anti-CD20 monoclonal antibody targeting B cells, has been shown to be effective in steroid-refractory, chronic graft-versus-host disease. However, most of the data were from small numbers of patients or retrospective analyses. We, therefore, conducted a multicenter phase II study to confirm the efficacy of this treatment strategy that targets B cells.
Design and methods: We diagnosed and evaluated chronic graft-versus-host disease according to the National Institute of Health criteria for clinical trials on this condition. The treatment consisted of weekly intravenous infusions of rituximab for 4 weeks followed by monthly rituximab for 4 months. We evaluated the patients' responses and monitored their disease activity until their final visit, which was on day 365. We also assessed the patients' subsequent quality of life and serum levels of B-cell-activating factor of the tumor necrosis factor family.
Results: Among 37 patients enrolled (median age, 29 years; range 8-57 years), 32 patients responded to rituximab with 8 complete and 24 partial responses. Twenty-one patients maintained their response for 1 year, so their steroid treatment was discontinued or its dose reduced (21/37, or 56.8%), and their scores representing quality of life were improved although these changes were not statistically significant. The responses were better for clinical manifestations of the skin, oral cavity and musculoskeletal system (response rate, 71.4-100%) than for other organs. However, infectious complications and primary disease relapse accounted for the majority of treatment failure. The pre-treatment serum level of B cell-activating factor of the tumor necrosis factor family was not associated with better treatment outcome (P=0.147).
Conclusions: Rituximab could improve clinical responses and quality of life of patients with steroid-refractory chronic graft-versus-host disease, although such patients may need active prophylaxis against infection.
Figures
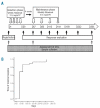
Figure 1.
(A) Treatment schedule and response evaluation. (B) The time to maximal response in 37 patients. The median time to maximal response was day 29, and the range was from day 0 (for non-responders) to day 252.
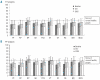
Figure 2.
(A) In the ITT analysis of quality of life, patients reported increased scores in six domains (RP, BP, GH, SF, RE and MH) although only the effects on RP and BP were statistically significant (P<0.05). (B) PP analysis, including only patients with complete follow-up through day 365 showed a tendency toward improvement in all domains of the SF36 quality of life questionnaire although not statistically significant. GH, general health perceptions; PF, physical function; MH, general mental health; RP, role function limitation due to physical problems; RE, role function limitation due to emotional problems; BP, bodily pain; VT, vitality; SF, social function; PCS, physical component summary; MCS, mental component summary
Figure 3.
Serial changes in serum BAFF and immunoglobulins. Serum BAFF increased continuously, but was inversely correlated with the sustained decrease in immune globulins.
Comment in
- B-cell-directed therapy for chronic graft-versus-host disease.
Jacobson CA, Ritz J. Jacobson CA, et al. Haematologica. 2010 Nov;95(11):1811-3. doi: 10.3324/haematol.2010.032227. Haematologica. 2010. PMID: 21037328 Free PMC article. No abstract available.
Similar articles
- B-cell-directed therapy for chronic graft-versus-host disease.
Jacobson CA, Ritz J. Jacobson CA, et al. Haematologica. 2010 Nov;95(11):1811-3. doi: 10.3324/haematol.2010.032227. Haematologica. 2010. PMID: 21037328 Free PMC article. No abstract available. - Corticosteroid-Free Primary Treatment of Chronic Extensive Graft-versus-Host Disease Incorporating Rituximab.
Solomon SR, Sizemore CA, Ridgeway M, Zhang X, Smith J, Brown S, Holland HK, Morris LE, Bashey A. Solomon SR, et al. Biol Blood Marrow Transplant. 2015 Sep;21(9):1576-82. doi: 10.1016/j.bbmt.2015.04.023. Epub 2015 May 16. Biol Blood Marrow Transplant. 2015. PMID: 25985915 - Effectiveness of subcutaneous low-dose alemtuzumab and rituximab combination therapy for steroid-resistant chronic graft-versus-host disease.
Gutiérrez-Aguirre CH, Cantú-Rodríguez OG, Borjas-Almaguer OD, González-Llano O, Jaime-Pérez JC, Solano-Genesta M, Gómez-Guijosa M, Mancias-Guerra C, Tarin L, Gómez-Almaguer D. Gutiérrez-Aguirre CH, et al. Haematologica. 2012 May;97(5):717-22. doi: 10.3324/haematol.2011.054577. Epub 2011 Dec 1. Haematologica. 2012. PMID: 22133770 Free PMC article. - Clinical utility of rituximab in chronic graft-versus-host disease.
Bates JS, Engemann AM, Hammond JM. Bates JS, et al. Ann Pharmacother. 2009 Feb;43(2):316-21. doi: 10.1345/aph.1L386. Epub 2009 Feb 3. Ann Pharmacother. 2009. PMID: 19193571 Review. - B cells in chronic graft-versus-host disease.
Sarantopoulos S, Blazar BR, Cutler C, Ritz J. Sarantopoulos S, et al. Biol Blood Marrow Transplant. 2015 Jan;21(1):16-23. doi: 10.1016/j.bbmt.2014.10.029. Epub 2014 Nov 12. Biol Blood Marrow Transplant. 2015. PMID: 25452031 Free PMC article. Review.
Cited by
- Therapeutic effect of budesonide/formoterol, montelukast and N-acetylcysteine for bronchiolitis obliterans syndrome after hematopoietic stem cell transplantation.
Kim SW, Rhee CK, Kim YJ, Lee S, Kim HJ, Lee JW. Kim SW, et al. Respir Res. 2016 May 26;17(1):63. doi: 10.1186/s12931-016-0380-1. Respir Res. 2016. PMID: 27229850 Free PMC article. - Prophylactic rituximab after allogeneic transplantation decreases B-cell alloimmunity with low chronic GVHD incidence.
Arai S, Sahaf B, Narasimhan B, Chen GL, Jones CD, Lowsky R, Shizuru JA, Johnston LJ, Laport GG, Weng WK, Benjamin JE, Schaenman J, Brown J, Ramirez J, Zehnder JL, Negrin RS, Miklos DB. Arai S, et al. Blood. 2012 Jun 21;119(25):6145-54. doi: 10.1182/blood-2011-12-395970. Epub 2012 May 4. Blood. 2012. PMID: 22563089 Free PMC article. Clinical Trial. - B-cell-mediated strategies to fight chronic allograft rejection.
Dalloul A. Dalloul A. Front Immunol. 2013 Dec 17;4:444. doi: 10.3389/fimmu.2013.00444. Front Immunol. 2013. PMID: 24381571 Free PMC article. Review. - Impact of Immune-Modulatory Drugs on Regulatory T Cell.
Furukawa A, Wisel SA, Tang Q. Furukawa A, et al. Transplantation. 2016 Nov;100(11):2288-2300. doi: 10.1097/TP.0000000000001379. Transplantation. 2016. PMID: 27490409 Free PMC article. Review. - Monogenic Immune Diseases Provide Insights Into the Mechanisms and Treatment of Chronic Graft-Versus-Host Disease.
Rozmus J. Rozmus J. Front Immunol. 2021 Feb 4;11:574569. doi: 10.3389/fimmu.2020.574569. eCollection 2020. Front Immunol. 2021. PMID: 33613511 Free PMC article. Review.
References
- Ratanatharathorn V, Ayash L, Lazarus HM, Fu J, Uberti JP. Chronic graft-versus-host disease: clinical manifestation and therapy. Bone Marrow Transplant. 2001;28(2):121–9. - PubMed
- Lee SJ, Vogelsang G, Flowers ME. Chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2003;9(4):215–33. - PubMed
- Vogelsang GB. How I treat chronic graft-versus-host disease. Blood. 2001;97(5):1196–201. - PubMed
- Koc S, Leisenring W, Flowers ME, Anasetti C, Deeg HJ, Nash RA, et al. Therapy for chronic graft-versus-host disease: a randomized trial comparing cyclosporine plus prednisone versus prednisone alone. Blood. 2002;100(1):48–51. - PubMed
- Arora M, Wagner JE, Davies SM, Blazar BR, Defor T, Enright H, et al. Randomized clinical trial of thalidomide, cyclosporine, and prednisone versus cyclosporine and prednisone as initial therapy for chronic graft-versus-host disease. Biol Blood Marrow Transplant. 2001;7(5):265–73. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources