Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma - PubMed (original) (raw)
Zoledronic acid impairs myeloid differentiation to tumour-associated macrophages in mesothelioma
J D Veltman et al. Br J Cancer. 2010.
Abstract
Background: Suppressive immune cells present in tumour microenvironments are known to augment tumour growth and hamper efficacy of antitumour therapies. The amino-bisphosphonate Zoledronic acid (ZA) is considered as an antitumour agent, as recent studies showed that ZA prolongs disease-free survival in cancer patients. The exact mechanism is a topic of debate; it has been suggested that ZA targets tumour-associated macrophages (TAMs).
Methods: We investigate the role of ZA on the myeloid differentiation to TAMs in murine mesothelioma in vivo and in vitro. Mice were intraperitoneally inoculated with a lethal dose of mesothelioma tumour cells and treated with ZA to determine the effects on myeloid differentiation and survival.
Results: We show that ZA impaired myeloid differentiation. Inhibition of myeloid differentiation led to a reduction in TAMs, but the number of immature myeloid cells with myeloid-derived suppressor cell (MDSC) characteristics was increased. In addition, ZA affects the phenotype of macrophages leading to reduced level of TAM-associated cytokines in the tumour microenvironment. No improvement of survival was observed.
Conclusion: We conclude that ZA leads to a reduction in macrophages and impairs polarisation towards an M2 phenotype, but this was associated with an increase in the number of immature myeloid cells, which might diminish the effects of ZA on survival.
Figures
Figure 1
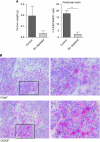
Depletion of macrophages inhibits tumour development. On day 1, mice were i.p. injected with a lethal dose of AC29 mesothelioma tumour cells and were treated twice with liposome-encapsulated clodronate (macrophage depletion) or liposome-encapsulated PBS (control) on days 5 and 10 after tumour injection (_n_=10). Twelve days after tumour injection, mice were killed and tumour weight was measured. All visible tumour material was excised from each mouse and data are expressed as wet weight (accuracy of 0.001 g). FACS analysis was performed to verify the effectiveness of macrophage depletion using liposome-encapsulated clodronate. Tumour biopsies were embedded in Tissue-Tek II and snap frozen in liquid nitrogen. Tissue sections (6 _μ_m) were analysed for the presence of macrophages. (A) Effect of macrophage depletion on tumour growth. Tumour growth was observed in all five mice treated with control liposomes; in contrast, only two out of five mice treated with macrophage-depleting liposomes developed visible tumour growth on day 12. Significant reduction in the percentage of F4/80+MHCII+ cells was found in the peritoneal cavity of macrophage-depleted mice (_P_=0.0015). Tumour weight was found to be lower in macrophage-depleted animals (_P_=0.077). (B) TAMs in murine mesothelioma. Tumour biopsies of control-treated mice showed infiltration of F4/80+ and CD206+ cells (magnification: upper, × 200; lower, × 400). **P<0.001.
Figure 2
ZA inhibits differentiation of myeloid cells in vitro. The effect of ZA on myeloid differentiation was determined by cultured bone marrow-derived cells with 0.5 _μ_g ml−1 M-CSF or RPMI containing 30% tumour supernatant. Cells were cultured for 6 days. ZA was added to the cultures on day 0 in different concentrations (0.03, 0.15 or 0.30 μ
M
). FACS analysis was performed daily. ZA inhibits the downregulation of Gr-1+ cells and the upregulation of F4/80+ and MHCII+ cells in a dose-dependent manner. Experiments were repeated and data of five individual experiments were then combined. No significant differences were observed in the number of cells between the different culture conditions. A significant difference was found in the percentage of immature myeloid cells and the percentage of macrophages after 6 days of culture in both, resulting in a higher number of immature cells and a lower number of macrophages in ZA culture conditions. (A) Bone marrow culture with M-CSF: immature myeloid cells, *_P_=0.004; **_P_=0.0001; ***P<0.0001; macrophages, *_P_=0.0003; **P<0.0001; ***_P_=0.005. (B) Bone marrow culture with 30% tumour supernatant: immature myeloid cells, *_P_=0.016; **_P_=0.0014; ***_P_=0.0025; *″_P_=0.015; **″_P_=0.036; macrophages, *_P_=0.002; **_P_=0.0011; ***_P_=0.006; *″_P_=0.0004, **″_P_=0.001.
Figure 3
ZA inhibits the upregulation of extracellular markers in vitro. Expression profiles of M-CSF and RPMI containing 30% tumour supernatant cultured cells were measured by FACS to determine the effect of ZA addition to the culture (0.5 _μ_g ml−1 M-CSF; 0.30 μ
M
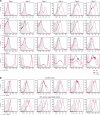
ZA was added on day 0). CD206 on macrophages was analysed to determine changes in macrophage phenotype. (A) M-CSF culture. F4/80, CD11c and MHCII were upregulated within 6 days. The immature myeloid marker Gr-1 was rapidly downregulated. The addition of ZA to the culture supernatant reduced the upregulation F4/80 and MHCII and CD11c, leading to a significant difference in MFI of these markers on day 6 of culture (_P_=0.003, 0.0023, 0.0003, respectively). As a consequence, the expression of Gr-1 was still high in a majority of the cells after 6 days of culture. (B) CD206 expression on macrophages (M-CSF culture and 30% tumour supernatant culture). After day 5, almost all F4/80+MHCII+ cells in the M-CSF culture expressed CD206. The upregulation of CD206 on cells cultured in the presence of tumour supernatant was more explicit. The addition of ZA to the cultures reduced the expression of CD206 on macrophages in both conditions and a significant reduction in the MFI of CD206 on macrophages after 6 days of culture (P<0.0001). Experiments were repeated several times under comparable conditions (_n_=5). Determination of the significance of peak shifts was based on calculation of the MFI.
Figure 4
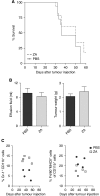
Treatment with ZA does not improve survival. Mice were divided into two groups (_n_=10 mice per group). Mice were treated daily with s.c. ZA (100 _μ_g kg−1, ∼2.5 _μ_g per mice) or PBS injections starting on day 5 after tumour injection. This dosing schedule was proven effective and non-toxic (Stathopoulos et al, 2008). Mice were killed when found profoundly ill. No significant improvement of survival was measured. (A) Kaplan–Meier survival curve. No significant differences in survival were observed between mice treated daily with s.c. injection of ZA compared with untreated mice (_P_=0.3675). (B) Malignant effusions and tumour weights. Tumour weight and the amount of malignant effusion were measured when mice were killed. The effusion fluid was removed from the peritoneal cavity by fine-needle aspiration and all visible tumour material was collected. No significant differences in tumour weight or the amount of malignant effusion were observed (_P_=0.42 and 0.61). (C) Myeloid cell types in the spleen of tumour-bearing mice. Long-term treatment effects were observed in the number of myeloid cells within the spleen of tumour-bearing mice, implicating that higher numbers of myeloid precursors and lower numbers of TAMs were detected in mice treated with ZA compared with untreated mice.
Figure 5
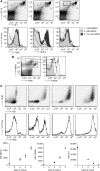
Identification of myeloid cells in tumour-bearing mice. To identify the effect of tumour growth on the recruitment of myeloid cells during tumour progression, mice were inoculated with tumour cells and killed on day 25 (_n_=12). (A) Identification of myeloid cell types in splenocytes of tumour-bearing mice. Immature myeloid cells could be divided into three groups, as described in the literature (Greifenberg et al, 2009). The Gr-1low-MDSC showed intermediate expression of F4/80, MHCII and CD206. F4/80 expression was found in MO-MDSC, but not in PMN-MDSC. A small number of MO-MDSC expressed high levels of F4/80 MHCII and CD206. MHCII and CD206 expressions were low in PMN-MDSC. (B) Identification of type I and type II macrophages in splenocytes of tumour-bearing mice. Two populations of macrophages could be identified; a high expression of CD206 was found in the membrane of the population with a high expression of F4/80 but lower expression of MHCII. (C) M-CSF culture of MO-MDSC-sorted cell fraction. MO-MDSC were sorted from splenocytes of tumour-bearing mice and cultured with 0.5 _μ_g ml−1 M-CSF. The expression of F4/80, MHCII, CD206 and Gr-1 were measured on consecutive days.
Figure 6
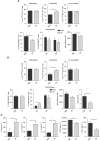
ZA changes M1:M2 ratio and increases MO-MDSC in tumour-bearing mice. Mice were i.p. inoculated with AC29 tumour cells and treated daily with s.c. injection of ZA. (100 _μ_g kg−1) or PBS as a control (_n_=6 each group). Mice were killed 25 days after tumour injection. The number of MDSC was analysed according to the subdivision as described in Figure 5. Macrophages were subdivided into M1 and M2 macrophages based on the co-expression of CD206, F4/80 and MHCII on the membrane. (A) Myeloid cell types in spleen of tumour-bearing mice. MO-MDSC were significantly increased in the spleen of ZA-treated animals (*_P_=0.0312). No difference was found in the percentage of PMN-MDSC and Gr-1low-MDSC (*_P_=0.77 and 0.75). The percentage of total macrophages in the spleen of ZA-treated mice was significantly lower compared with untreated mice (*_P_=0.0091). In the spleen of tumour-bearing mice, although not significant there was a trend towards a reduction in both M1 and M2 macrophages in ZA-treated mice. In addition, ZA treatment significantly lowers the MFI of CD206 on M2 macrophages (*_P_=0.0095). (B) Myeloid cell types in effusion fluid of tumour-bearing mice. MO-MDSCs were significantly increased in the effusion fluid of ZA-treated animals (*_P_=0.034). No difference was found in the percentage of PMN-MDSC and Gr-1low-MDSC or macrophages (*_P_=0.72 and 0.74). A significant increase in M1 macrophages was found (*_P_=0.035), and also an increase was found in the number of M2 macrophages (*_P_=0.33). ZA shifts the balance, leading to a significant difference in the ratio of M1:M2 macrophages (*_P_=0.011); a trend towards a lower MFI of CD206 was observed (*P_=0.114). (C) Cytokines in effusion fluid of tumour-bearing mice. ELISA was performed on effusion fluid of tumour-bearing mice treated with ZA or PBS as a control. A significant increase in IL-6, IL-12 and IL-1_β was found in ZA-treated mice (*_P_=0.049, 0.042 and 0.005, respectively). A significant reduction in VEGF and CCL-2 (MCP-1) expressions was found in ZA-treated mice (*_P_=0.05 and 0.039).
Similar articles
- Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway.
Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Mönkkönen H, Boccadoro M, Forni G, Musiani P, Bosia A, Cavallo F, Massaia M. Coscia M, et al. J Cell Mol Med. 2010 Dec;14(12):2803-15. doi: 10.1111/j.1582-4934.2009.00926.x. J Cell Mol Med. 2010. PMID: 19818098 Free PMC article. - Zoledronic acid impairs stromal reactivity by inhibiting M2-macrophages polarization and prostate cancer-associated fibroblasts.
Comito G, Pons Segura C, Taddei ML, Lanciotti M, Serni S, Morandi A, Chiarugi P, Giannoni E. Comito G, et al. Oncotarget. 2017 Jan 3;8(1):118-132. doi: 10.18632/oncotarget.9497. Oncotarget. 2017. PMID: 27223431 Free PMC article. - The effect of zoledronic acid on the function and differentiation of myeloid cells.
Wolf AM, Rumpold H, Tilg H, Gastl G, Gunsilius E, Wolf D. Wolf AM, et al. Haematologica. 2006 Sep;91(9):1165-71. Haematologica. 2006. PMID: 16956814 - Direct antitumour activity of zoledronic acid: preclinical and clinical data.
Bosch-Barrera J, Merajver SD, Menéndez JA, Van Poznak C. Bosch-Barrera J, et al. Clin Transl Oncol. 2011 Mar;13(3):148-55. doi: 10.1007/s12094-011-0634-9. Clin Transl Oncol. 2011. PMID: 21421459 Review. - Tumour macrophages as potential targets of bisphosphonates.
Rogers TL, Holen I. Rogers TL, et al. J Transl Med. 2011 Oct 17;9:177. doi: 10.1186/1479-5876-9-177. J Transl Med. 2011. PMID: 22005011 Free PMC article. Review.
Cited by
- Myeloid-derived cells are key targets of tumor immunotherapy.
Medina-Echeverz J, Aranda F, Berraondo P. Medina-Echeverz J, et al. Oncoimmunology. 2014 Apr 15;3:e28398. doi: 10.4161/onci.28398. eCollection 2014. Oncoimmunology. 2014. PMID: 25050208 Free PMC article. Review. - Tumor-Derived GM-CSF Promotes Granulocyte Immunosuppression in Mesothelioma Patients.
Khanna S, Graef S, Mussai F, Thomas A, Wali N, Yenidunya BG, Yuan C, Morrow B, Zhang J, Korangy F, Greten TF, Steinberg SM, Stetler-Stevenson M, Middleton G, De Santo C, Hassan R. Khanna S, et al. Clin Cancer Res. 2018 Jun 15;24(12):2859-2872. doi: 10.1158/1078-0432.CCR-17-3757. Epub 2018 Mar 30. Clin Cancer Res. 2018. PMID: 29602801 Free PMC article. - Atorvastatin promotes the expansion of myeloid-derived suppressor cells and attenuates murine colitis.
Lei A, Yang Q, Li X, Chen H, Shi M, Xiao Q, Cao Y, He Y, Zhou J. Lei A, et al. Immunology. 2016 Dec;149(4):432-446. doi: 10.1111/imm.12662. Epub 2016 Sep 20. Immunology. 2016. PMID: 27548304 Free PMC article. - Anti-tumour strategies aiming to target tumour-associated macrophages.
Tang X, Mo C, Wang Y, Wei D, Xiao H. Tang X, et al. Immunology. 2013 Feb;138(2):93-104. doi: 10.1111/imm.12023. Immunology. 2013. PMID: 23113570 Free PMC article. Review. - Macrophage depletion by free bisphosphonates and zoledronate-loaded red blood cells.
Sabatino R, Antonelli A, Battistelli S, Schwendener R, Magnani M, Rossi L. Sabatino R, et al. PLoS One. 2014 Jun 26;9(6):e101260. doi: 10.1371/journal.pone.0101260. eCollection 2014. PLoS One. 2014. PMID: 24968029 Free PMC article.
References
- Allavena P, Sica A, Garlanda C, Mantovani A (2008a) The Yin–Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol Rev 222: 155–161 - PubMed
- Allavena P, Sica A, Solinas G, Porta C, Mantovani A (2008b) The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66: 1–9 - PubMed
- Boissier S, Ferreras M, Peyruchaud O, Magnetto S, Ebetino FH, Colombel M, Delmas P, Delaisse JM, Clezardin P (2000) Bisphosphonates inhibit breast and prostate carcinoma cell invasion, an early event in the formation of bone metastases. Cancer Res 60: 2949–2954 - PubMed
- Boissier S, Magnetto S, Frappart L, Cuzin B, Ebetino FH, Delmas PD, Clezardin P (1997) Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res 57: 3890–3894 - PubMed
- Bronte V, Serafini P, Apolloni E, Zanovello P (2001) Tumor-induced immune dysfunctions caused by myeloid suppressor cells. J Immunother 24: 431–446 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical