Involvement of tissue plasminogen activator in stress responsivity during acute cocaine withdrawal in mice - PubMed (original) (raw)
Involvement of tissue plasminogen activator in stress responsivity during acute cocaine withdrawal in mice
Yan Zhou et al. Stress. 2010 Nov.
Abstract
There is evidence that increased release of corticotropin-releasing factor (CRF) in the central nucleus of the amygdala (CeA) contributes to stress responsivity during cocaine withdrawal (WD). Recent studies suggest that tissue plasminogen activator (tPA) in the CeA is a downstream effector protein for CRF after acute "binge" cocaine administration. The purpose of this study was to determine if tPA modulates cocaine WD-induced stress responsivity. Wild-type (WT) and tPA-deficient (tPA - / - ) mice were subjected to chronic (14 days) "binge" cocaine (45 mg/kg per day) or its acute (1 day) WD. Extracellular tPA activity, CRF mRNA levels, and plasma corticosterone (CORT) levels were measured in tPA - / - and WT mice. Extracellular tPA activity was reduced by 50% in the CeA and medial amygdala of WT mice after chronic cocaine and returned to basal levels after acute WD. Unlike WT mice, tPA - / - mice did not display elevated amygdalar CRF mRNA levels during cocaine WD. In comparison to WT mice, tPA - / - mice showed a blunted plasma CORT response during acute WD. These results demonstrate that tPA activity in the amygdala (Amy) is altered by chronic cocaine exposure, and further suggest an involvement of tPA in modulating amygdalar CRF stress responsive system and hypothalamic-pituitary-adrenal axis in response to acute cocaine WD.
Conflict of interest statement
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures
Figure 1
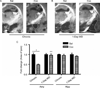
Extracellular tPA activity in the Amy and Hipp of WT mice was measured by in situ zymography 30 min after 14 days of chronic “binge” pattern cocaine administration (Chronic Coc) and 1 day after 14 days of Chronic Coc as 1-day WD (A,B). tPA activity (indicated by dark lytic zones) in the CeA and MeA (A) was quantified by measuring the area of lysis outlined by dashed white line (C). Data in graph C are presented as mean ± SEM. tPA activity was significantly decreased in the CeA and MeA of WT mice 30 min after 14 days of chronic “binge” cocaine (Chronic Coc), compared to those of saline-injected mice (*p < 0.05). Significant differences are also found between Chronic Coc and 1-day WD groups (++p < 0.01) (n = 3–4/group). In the hippocampus, tPA activity remained unchanged at both time points examined (C).
Figure 2
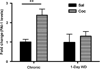
PAI-1 levels in the Amy of WT mice were examined by ELISA 30 min after 14 days of chronic “binge” pattern cocaine administration (Chronic Coc) and 1-day after 14 days of Chronic Coc as 1-day WD. Data in the graph are presented as mean ± SEM. PAI-1 levels were significantly enhanced in cocaine-treated animals 30 min after 14 days of chronic “binge” cocaine (Chronic Coc), compared to those of their saline- injected counterparts (**p < 0.01) (n = 3–4/group).
Figure 3
mRNA levels of corticotropin-releasing factor (CRF) (A,C) and CRF1 receptor (B,D) in the Amy of WT and tPA-deficient (tPA −/−) mice were examined 30 min after 14 days of chronic “binge” pattern cocaine administration (Chronic Coc) and 1 day after 14 days of Chronic Coc as 1-day WD. Data in the graph are presented as mean ± SEM. (A) In the Amy of WT mice, significant differences are indicated: +p < 0.05 between 1-day WD vs. chronic cocaine (Chronic Coc) groups; *p = 0.06 between 1-day WD from cocaine (1-day WD Coc) and 1-day WD from saline (1-day WD Sal) groups. (B) No significant differences in CRF1 receptor mRNA levels in WT mice were observed at either of the time points examined. (C,D) No significant differences in mRNA levels of either CRF or CRF1 receptor in tPA −/− mice were observed at either of the time points examined (n = 4–6/group).
Figure 4
Plasma CORT levels in WT (A) and tPA-deficient (tPA −/−) mice (B) were examined 30 min after 14 days of chronic “binge” pattern cocaine administration (Chronic Coc) and 1 day after 14 days of Chronic Coc (1-day WD). Data in the graph are presented as mean ± SEM. (A) In WT mice, significant differences are indicated: **p < 0.01 between chronic cocaine (Chronic Coc) vs. saline (Chronic Sal); ##p < 0.01 between 1-day acute WD (1-day WD) from cocaine vs. saline control (n = 5–8/group). (B) In tPA −/− mice, significant differences are indicated: *p < 0.05 between chronic cocaine (Chronic Coc) vs. saline (Chronic Sal).
Similar articles
- Increased CRH mRNA levels in the rat amygdala during short-term withdrawal from chronic 'binge' cocaine.
Zhou Y, Spangler R, Ho A, Kreek MJ. Zhou Y, et al. Brain Res Mol Brain Res. 2003 May 26;114(1):73-9. doi: 10.1016/s0169-328x(03)00139-6. Brain Res Mol Brain Res. 2003. PMID: 12782395 - Amygdalar vasopressin mRNA increases in acute cocaine withdrawal: evidence for opioid receptor modulation.
Zhou Y, Bendor JT, Yuferov V, Schlussman SD, Ho A, Kreek MJ. Zhou Y, et al. Neuroscience. 2005;134(4):1391-7. doi: 10.1016/j.neuroscience.2005.05.032. Neuroscience. 2005. PMID: 16039786 - Neurobiology of stress and cocaine addiction. Studies on corticotropin-releasing factor in rats, monkeys, and humans.
Sarnyai Z. Sarnyai Z. Ann N Y Acad Sci. 1998 Jun 30;851:371-87. doi: 10.1111/j.1749-6632.1998.tb09011.x. Ann N Y Acad Sci. 1998. PMID: 9668628 Review.
Cited by
- The epigenetic landscape of alcoholism.
Krishnan HR, Sakharkar AJ, Teppen TL, Berkel TD, Pandey SC. Krishnan HR, et al. Int Rev Neurobiol. 2014;115:75-116. doi: 10.1016/B978-0-12-801311-3.00003-2. Int Rev Neurobiol. 2014. PMID: 25131543 Free PMC article. Review. - PKCδ-positive GABAergic neurons in the central amygdala exhibit tissue-type plasminogen activator: role in the control of anxiety.
Douceau S, Lemarchand E, Hommet Y, Lebouvier L, Joséphine C, Bemelmans AP, Maubert E, Agin V, Vivien D. Douceau S, et al. Mol Psychiatry. 2022 Apr;27(4):2197-2205. doi: 10.1038/s41380-022-01455-4. Epub 2022 Feb 10. Mol Psychiatry. 2022. PMID: 35145231 - Neuroendocrine Targeting of Tissue Plasminogen Activator (t-PA).
Parmer RJ, Gong Y, Yoo SH, Miles LA. Parmer RJ, et al. J Neurol Disord Stroke. 2020;7(1):1153. Epub 2020 Feb 12. J Neurol Disord Stroke. 2020. PMID: 32549050 Free PMC article. - Targeting NMDA Receptors at the Neurovascular Unit: Past and Future Treatments for Central Nervous System Diseases.
Seillier C, Lesept F, Toutirais O, Potzeha F, Blanc M, Vivien D. Seillier C, et al. Int J Mol Sci. 2022 Sep 7;23(18):10336. doi: 10.3390/ijms231810336. Int J Mol Sci. 2022. PMID: 36142247 Free PMC article. Review. - Glucocorticoid-Responsive Tissue Plasminogen Activator (tPA) and Its Inhibitor Plasminogen Activator Inhibitor-1 (PAI-1): Relevance in Stress-Related Psychiatric Disorders.
Mennesson M, Revest JM. Mennesson M, et al. Int J Mol Sci. 2023 Feb 24;24(5):4496. doi: 10.3390/ijms24054496. Int J Mol Sci. 2023. PMID: 36901924 Free PMC article. Review.
References
- Ambrosio E, Sharpe LG, Pilotte NS. Regional binding to corticotropin releasing factor receptors in brain of rats exposed to chronic cocaine and cocaine withdrawal. Synapse. 1997;25:272–276. - PubMed
- Aouizerate B, Ho A, Schluger JH, Perret G, Borg L, Le Moal M, Piazza PV, Kreek MJ. Glucocroticoid negative feedback in methadone-maintained former heroin addicts with ongoing cocaine dependence: Dose-response to dexamethasone suppression. Addict Biol. 2006;11:84–96. - PubMed
- Bahi A, Dreyer JL. Overexpression of plasminogen activators in the nucleus accumbens enhances cocaine-, amphetamine- and morphine-induced reward and behavioral sensitization. Genes Brain Behav. 2008;7:244–256. - PubMed
- Baranes D, Lederfein D, Huang YY, Chen M, Bailey CH, Kandel ER. Tissue plasminogen activator contributes to the late phase of LTP and to synaptic growth in the hippocampal mossy fiber pathway. Neuron. 1998;21:813–825. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS035704/NS/NINDS NIH HHS/United States
- P60 DA005130/DA/NIDA NIH HHS/United States
- NS035704/NS/NINDS NIH HHS/United States
- AA014630/AA/NIAAA NIH HHS/United States
- R01 AA014630/AA/NIAAA NIH HHS/United States
- DA-P60-05130/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources