Cerebral microcirculation is impaired during sepsis: an experimental study - PubMed (original) (raw)
Cerebral microcirculation is impaired during sepsis: an experimental study
Fabio Silvio Taccone et al. Crit Care. 2010.
Abstract
Introduction: Pathophysiology of brain dysfunction due to sepsis remains poorly understood. Cerebral microcirculatory alterations may play a role; however, experimental data are scarce. This study sought to investigate whether the cerebral microcirculation is altered in a clinically relevant animal model of septic shock.
Methods: Fifteen anesthetized, invasively monitored, and mechanically ventilated female sheep were allocated to a sham procedure (n = 5) or sepsis (n = 10), in which peritonitis was induced by intra-abdominal injection of autologous faeces. Animals were observed until spontaneous death or for a maximum of 20 hours. In addition to global hemodynamic assessment, the microcirculation of the cerebral cortex was evaluated using Sidestream Dark-Field (SDF) videomicroscopy at baseline, 6 hours, 12 hours and at shock onset. At least five images of 20 seconds each from separate areas were recorded at each time point and stored under a random number to be analyzed, using a semi-quantitative method, by an investigator blinded to time and condition.
Results: All septic animals developed a hyperdynamic state associated with organ dysfunction and, ultimately, septic shock. In the septic animals, there was a progressive decrease in cerebral total perfused vessel density (from 5.9 ± 0.9 at baseline to 4.8 ± 0.7 n/mm at shock onset, P = 0.009), functional capillary density (from 2.8 ± 0.4 to 2.1 ± 0.7 n/mm, P = 0.049), the proportion of small perfused vessels (from 95 ± 3 to 85 ± 8%, P = 0.02), and the total number of perfused capillaries (from 22.7 ± 2.7 to 17.5 ± 5.2 n/mm, P = 0.04). There were no significant changes in microcirculatory flow index over time. In sham animals, the cerebral microcirculation was unaltered during the study period.
Conclusions: In this model of peritonitis, the cerebral microcirculation was impaired during sepsis, with a significant reduction in perfused small vessels at the onset of septic shock. These alterations may play a role in the pathogenesis of septic encephalopathy.
Figures
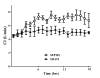
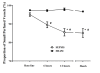
Figure 1
Evolution over time of cardiac index (CI) in sham (n = 5) and septic (n = 10) animals.
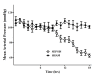
Figure 2
Evolution over time of mean arterial pressure (MAP) in sham (n = 5) and septic (n = 10) animals.
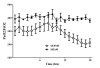
Figure 3
Evolution over time of PaO2/FiO2 ratio in sham (n = 5) and septic (n = 10) animals.
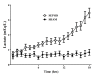
Figure 4
Evolution over time of lactate levels in sham (n = 5) and septic (n = 10) animals.
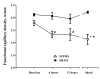
Figure 5
Changes in cerebral functional capillary density (FCD) in the septic (n = 10) and the sham (n = 5) animals. Data are presented as mean ± SD. ANOVA analysis for FCD: P = 0.049 (time, sepsis group) and P < 0.001 (group). ANOVA analysis for PSPV: P = 0.02 (time, sepsis group) and P < 0.001 (group). _P_-value <.05 vs. baseline (*) or vs. sham (#) in post-hoc Bonferroni correction.
Figure 6
Changes in proportion of small perfused vessels (PSPV) in the septic (n = 10) and the sham (n = 5) animals. Data are presented as mean ± SD. ANOVA analysis for FCD: P = 0.049 (time, sepsis group) and P < 0.001 (group). ANOVA analysis for PSPV: P = 0.02 (time, sepsis group) and P < 0.001 (group). _P-_value <.05 vs. baseline (*) or vs. sham (#) in post-hoc Bonferroni correction.
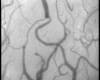
Figure 7
Digital photomicrographs of the cerebral cortical microcirculation of a septic animal at baseline.
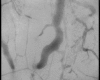
Figure 8
Digital photomicrographs of the cerebral cortical microcirculation of a septic animal at shock onset.
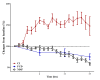
Figure 9
Correlation between microcirculation and global hemodynamics. Changes from baseline (100%) of cardiac index (CI; red circles), mean arterial pressure (MAP; white circles) and functional capillary density (FCD; blue circles) over the study period. Changes in FCD appear to occur earlier than significant changes in MAP and already during the hyperdynamic phase.
Comment in
- Septic-associated encephalopathy--everything starts at a microlevel.
Sharshar T, Polito A, Checinski A, Stevens RD. Sharshar T, et al. Crit Care. 2010;14(5):199. doi: 10.1186/cc9254. Epub 2010 Sep 29. Crit Care. 2010. PMID: 21067627 Free PMC article. Review.
Similar articles
- Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis.
Taccone FS, Su F, De Deyne C, Abdellhai A, Pierrakos C, He X, Donadello K, Dewitte O, Vincent JL, De Backer D. Taccone FS, et al. Crit Care Med. 2014 Feb;42(2):e114-22. doi: 10.1097/CCM.0b013e3182a641b8. Crit Care Med. 2014. PMID: 24196192 - Micro- and Macrocirculatory Changes During Sepsis and Septic Shock in a Rat Model.
Hua T, Wu X, Wang W, Li H, Bradley J, Peberdy MA, Ornato JP, Tang W. Hua T, et al. Shock. 2018 May;49(5):591-595. doi: 10.1097/SHK.0000000000000954. Shock. 2018. PMID: 28731900 - Similar Microcirculatory Alterations in Patients with Normodynamic and Hyperdynamic Septic Shock.
Edul VS, Ince C, Vazquez AR, Rubatto PN, Espinoza ED, Welsh S, Enrico C, Dubin A. Edul VS, et al. Ann Am Thorac Soc. 2016 Feb;13(2):240-7. doi: 10.1513/AnnalsATS.201509-606OC. Ann Am Thorac Soc. 2016. PMID: 26624559 - Brain perfusion in sepsis.
Taccone FS, Scolletta S, Franchi F, Donadello K, Oddo M. Taccone FS, et al. Curr Vasc Pharmacol. 2013 Mar 1;11(2):170-86. doi: 10.2174/1570161111311020007. Curr Vasc Pharmacol. 2013. PMID: 23506496 Review. - Sepsis/septic shock: participation of the microcirculation: an abbreviated review.
Hinshaw LB. Hinshaw LB. Crit Care Med. 1996 Jun;24(6):1072-8. doi: 10.1097/00003246-199606000-00031. Crit Care Med. 1996. PMID: 8681576 Review.
Cited by
- Underestimated Subsequent Sensorineural Hearing Loss after Septicemia.
Cheng CG, Chen YH, Chang YH, Lin HC, Chin PW, Lin YY, Yung MC, Cheng CA. Cheng CG, et al. Medicina (Kaunas). 2023 Oct 26;59(11):1897. doi: 10.3390/medicina59111897. Medicina (Kaunas). 2023. PMID: 38003946 Free PMC article. - Early course of microcirculatory perfusion in eye and digestive tract during hypodynamic sepsis.
Pranskunas A, Pilvinis V, Dambrauskas Z, Rasimaviciute R, Planciuniene R, Dobozinskas P, Veikutis V, Vaitkaitis D, Boerma EC. Pranskunas A, et al. Crit Care. 2012 May 15;16(3):R83. doi: 10.1186/cc11341. Crit Care. 2012. PMID: 22587828 Free PMC article. - Can infections cause Alzheimer's disease?
Mawanda F, Wallace R. Mawanda F, et al. Epidemiol Rev. 2013;35(1):161-80. doi: 10.1093/epirev/mxs007. Epub 2013 Jan 24. Epidemiol Rev. 2013. PMID: 23349428 Free PMC article. Review. - Common molecular and pathophysiological underpinnings of delirium and Alzheimer's disease: molecular signatures and therapeutic indications.
Mosharaf MP, Alam K, Gow J, Mahumud RA, Mollah MNH. Mosharaf MP, et al. BMC Geriatr. 2024 Aug 29;24(1):716. doi: 10.1186/s12877-024-05289-3. BMC Geriatr. 2024. PMID: 39210294 Free PMC article. - Angiotensin 1-7 in an experimental septic shock model.
Garcia B, Su F, Manicone F, Dewachter L, Favory R, Khaldi A, Moiroux-Sahroui A, Moreau A, Herpain A, Vincent JL, Creteur J, Taccone FS, Annoni F. Garcia B, et al. Crit Care. 2023 Mar 13;27(1):106. doi: 10.1186/s13054-023-04396-8. Crit Care. 2023. PMID: 36915144 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical