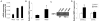High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia - PubMed (original) (raw)
High-mobility group box 1 promotes metalloproteinase-9 upregulation through Toll-like receptor 4 after cerebral ischemia
Jianhua Qiu et al. Stroke. 2010 Sep.
Abstract
Background and purpose: HMGB1 is a nuclear protein and an alarmin that signals cell damage in response to injury. It is believed that after release from injured cells, HMGB1 binds to its receptors to stimulate cross-talk among cells and to drive components of the inflammatory cascade. This study was intended to investigate the role of extracellular HMGB1 in ischemic stroke by examining the response of the zymogen matrix metalloproteinase-9 (MMP-9) to HMGB1 in vivo and in vitro.
Methods: Toll-like receptor 2 (TLR2), TLR4, receptor for advanced glycation endproducts (RAGE), and MMP-9 expression was examined using quantitative RT-PCR in primary cultured neurons, astrocytes, and mouse brain after HMGB1 addition. MMP-9 expression/activity was examined using zymography. Middle cerebral artery occlusion was induced for 60 minutes using a filament model.
Results: TLR4 is constitutively expressed in neurons, astrocytes, and mouse brain. HMGB1 addition to neuronal and glial cell cultures caused MMP-9 upregulation in a dose- and time-dependent manner. Lack of TLR4 function attenuated MMP-9 expression induced by HMGB1 in vitro. After striatal microinjection of HMGB1, MMP-9 was upregulated, and the response was independent of tumor necrosis factor-alpha. Interestingly, MMP-9 upregulation was reduced in TLR4 missense mutant mice after ischemia compared with wild-type controls, as was infarct volume.
Conclusions: Our results suggest that HMGB1 triggers MMP-9 upregulation in neurons and astrocytes predominantly via TLR4 after cerebral ischemia. Hence, targeting HMGB1/TLRs signaling pathway may reduce the acute inflammatory response and reduce tissue damage in cerebral ischemia.
Figures
1
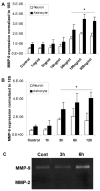
HMGB1 induces MMP-9 expression in neurons and astrocytes. Primary cultured neurons and astrocytes were treated with different doses of HMGB1 (A; 6h incubation) for various incubation periods (B; 100ng/ml of HMGB1). MMP-9 expression in the cells increases in a time and dose-dependent manner. C: Gel zymography confirms MMP-9 upregulation in the conditioned media from astrocytes. No MMP-2 is detected in the media. The data are the means±SD. *: p<0.05.
2
Expression of TLR4, TLR2 and RAGE, HMGB1 putative receptors in cultured neurons and astrocytes. Total RNA was isolated from primary cultured neurons and astrocytes. TLR4, TLR2 and RAGE mRNAs were analyzed by quantitative real time PCR. The expression levels were normalized to 18S expression. The data are shown as the mean±SD. Y-axis is a log scale.
3
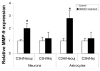
HMGB1-induced MMP-9 upregulation is attenuated in TLR4 mutant neurons and astrocytes. Neurons and astrocytes were isolated from either C3H/Heouj (wild-type) or C3H/Hej (mutant) mice and treated with 100ng/ml HMGB1 for 6 hours. MMP-9 expression was analyzed by real time PCR. The data show means±SD relative to control. *: <0.01.
4
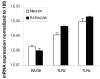
HMGB1 upregulates MMP-9 in mouse brain. A: expressions of RAGE, TLR2 and TLR4 in normal C57B/6 mice examined by real time PCR. The expression level is normalized to 18S expression. Y-axis is a log scale. B: Recombinant HMGB1 (100ng) or saline (control) was injected into C57B/6 mouse brains and MMP-9 mRNA was examined 6 hours after injection by real time PCR. The real time PCR data are the means±SD. *: p<0.01. C: recombinant HMGB1 (100ng) or saline control was injected into TNFα wild-type (WT) or knockout mouse brains and MMP-9 mRNA expression was assayed 6 hours after injection by gel zymography. No significant difference was observed between wild-type and knockout mice. D: 100ng of HMGB1 or saline (control) were injected into C3H mouse brains and mRNA of MMP-9 was analyzed by real time PCR. **: p<0.01.
5
MMP-9 expression and infarct volume after MCAO. A: The wild-type mice were subjected to 1h MCAO and sacrificed 6 hours after reperfusion. MMP-9 expression in cerebral cortex was examined by immunostaining. Upper left image shows representative region of MMP-9 examined in neurons (NeuN) and astrocytes (GFAP). No significant MMP-9 expression was detected in normal brains (lower left image). Scale bar: 50μm. B: MMP-9 expression examined by zymography was attenuated in cerebral cortex of TLR4 mutants (C3H/Hej) compared to wild-type mice (C3H/Heouj) after MCAO. C: Infarct volume was reduced in C3H/Hej mice examined by TTC staining 24h after MCAO. Data are means±SD. *: p<0.05.
References
- Lo EH, Dalkara T, Moskowitz MA. Mechanisms, challenges and opportunities in stroke. Nat Rev Neurosci. 2003;4:399–415. -PubMed
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: New opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. -PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 NS010828/NS/NINDS NIH HHS/United States
- R01 NS056458/NS/NINDS NIH HHS/United States
- R01 NS048422/NS/NINDS NIH HHS/United States
- R21 NS056214/NS/NINDS NIH HHS/United States
- P30 NS045776/NS/NINDS NIH HHS/United States
- R37 NS037074/NS/NINDS NIH HHS/United States
- 5R01NS010828-33/NS/NINDS NIH HHS/United States
- R01NS48422/NS/NINDS NIH HHS/United States
- K08 NS049241/NS/NINDS NIH HHS/United States
- P01 NS055104/NS/NINDS NIH HHS/United States
- K08NS049241/NS/NINDS NIH HHS/United States
- P30-NS045776/NS/NINDS NIH HHS/United States
- R37NS37074/NS/NINDS NIH HHS/United States
- R01NS56458/NS/NINDS NIH HHS/United States
- R21NS056214/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous