Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases - PubMed (original) (raw)
. 2010 Jul 27;8(7):e1000439.
doi: 10.1371/journal.pbio.1000439.
John R Walker, Valérie Campagna-Slater, Patrick J Finerty, Ragika Paramanathan, Galina Bernstein, Farrell MacKenzie, Wolfram Tempel, Hui Ouyang, Wen Hwa Lee, Elan Z Eisenmesser, Sirano Dhe-Paganon
Affiliations
- PMID: 20676357
- PMCID: PMC2911226
- DOI: 10.1371/journal.pbio.1000439
Structural and biochemical characterization of the human cyclophilin family of peptidyl-prolyl isomerases
Tara L Davis et al. PLoS Biol. 2010.
Abstract
Peptidyl-prolyl isomerases catalyze the conversion between cis and trans isomers of proline. The cyclophilin family of peptidyl-prolyl isomerases is well known for being the target of the immunosuppressive drug cyclosporin, used to combat organ transplant rejection. There is great interest in both the substrate specificity of these enzymes and the design of isoform-selective ligands for them. However, the dearth of available data for individual family members inhibits attempts to design drug specificity; additionally, in order to define physiological functions for the cyclophilins, definitive isoform characterization is required. In the current study, enzymatic activity was assayed for 15 of the 17 human cyclophilin isomerase domains, and binding to the cyclosporin scaffold was tested. In order to rationalize the observed isoform diversity, the high-resolution crystallographic structures of seven cyclophilin domains were determined. These models, combined with seven previously solved cyclophilin isoforms, provide the basis for a family-wide structure:function analysis. Detailed structural analysis of the human cyclophilin isomerase explains why cyclophilin activity against short peptides is correlated with an ability to ligate cyclosporin and why certain isoforms are not competent for either activity. In addition, we find that regions of the isomerase domain outside the proline-binding surface impart isoform specificity for both in vivo substrates and drug design. We hypothesize that there is a well-defined molecular surface corresponding to the substrate-binding S2 position that is a site of diversity in the cyclophilin family. Computational simulations of substrate binding in this region support our observations. Our data indicate that unique isoform determinants exist that may be exploited for development of selective ligands and suggest that the currently available small-molecule and peptide-based ligands for this class of enzyme are insufficient for isoform specificity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
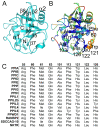
Figure 1. Structural elements of the cyclophilin fold and the definition of the active surface of PPIA.
(A) Secondary structural elements of PPIA in ribbon representation, with key structural elements labeled. All structural outputs were generated using PyMol unless otherwise noted. (B) Consurf representation of sequence conservation within the human cyclophilin family; residues that compose the active surface of the cyclophilin family are labeled . (C) Comparison of the sequences that define the active surface of the PPIase domain. Residue numbering corresponds to PPIA.
Figure 2. Structural coverage of the human cyclophilin family.
Cartoon representation of the novel experimental and modeled structures of human cyclophilins associated with this manuscript. Only the isomerase domain is shown. The previously determined structure of PPIA is shown as a reference point, and loop regions discussed in the text are outlined with dotted ovals and labeled. The structures of RanBP2, PPIL6, and PPIL4 are marked with an asterisk, as they are derived from homology modeling using the Phyre server and do not represent experimentally derived data. For crystallographic data concerning the structures shown here, refer to Table S1.
Figure 3. The structural consequences of substitutions in the cyclophilin active site.
The residues described in Figure 1 are shown in stick representation for the divergent family members PPIA, NKTR, PPIL2, and SDCCAG-10. Note the orientation of the divergent residues Tyr389 in PPIL2 and Glu122 in SDCCAG-10 relative to Trp121 in PPIA or His132 in NKTR.
Figure 4. The S2 “gatekeeper” region of the human cyclophilins.
(A) The definition of the S1′ pocket and S2 pocket is shown by depiction of a complex between PPIA and the tetrapeptide suc-AGPF-pNA (PDB 1ZKF). Surface representation of charges calculated within PyMol is shown, colored blue for basic and red for acidic regions. Residues around the active site and the S2 pocket are labeled according to PPIA numbering. (B) Sequence diversity of the gatekeeper residues in two PPIase domain structures. An “occluded” cyclophilin (NKTR) is shown in comparison to PPIA. As shown in Figure 5, these substitutions lead to diverse size and charge properties in this region of the cyclophilin active surfaces. (C) Comparison of the amino acids that define the S2 pocket of the PPIase domain. Residue numbering corresponds to PPIA.
Figure 5. The diverse surfaces of the human cyclophilins.
Surfaces of the human cyclophilins are shown colored by qualitative electrostatic potential. The scales of the potentials are all roughly the same (average potential: ±65 kBT/e) and range from ±56 kBT/e for PPIG to ±81kBT/e for PPIL4; all surfaces were calculated using the protein contact potential function in PyMol. As discussed in the text, the surfaces have been generally divided into those with neutral or mixed charge character surrounding the S2 pocket; those with largely acidic character around the S2 pocket; and those whose gatekeeper residue identities lead to occlusion of the S2 pocket.
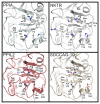
Figure 6. Peptide:protein simulations for five members of the cyclophilin family.
At the top are detailed results for PPIA. Simulations were set up with the structure of PPIA (PDB 1AK4) and with 400 peptides corresponding to the sequences X-Z-G-P, where X and Z are each of the possible combinations of the naturally occurring 20 amino acids. Gatekeeper residues were allowed flexibility during the simulations and are noted for each family member. The middle two panels are graphical representations of the PPIA results. On the left is a scatter plot with the energy metric on the y-axis and the distance metric on the x-axis. The lower left quadrant is where the highest-scoring peptide combinations are plotted (greatest negative energy and closest interaction with the S2 pocket). The color of each spot in the plot corresponds to the hydrogen bonding potential between that particular peptide and PPIA, with red indicating greater values (nine for the PPIA simulation) and purple indicating lesser values (two for the PPIA simulation). Scatter plots for all other simulations are found in Figure S6. In the panel on the right, the identity of the residue at position P3 is plotted along the x-axis, and the identity of the residue at the P2 position is plotted along the y-axis. The general chemical classification for each residue set is indicated. At the intersection of each x, y point is a square representing the binding energy and distance metrics. Red indicates greater binding energy for that x, y pair; purple indicates lesser energy. Value ranges for PPIA were −5.5 (red) to 4.3 (purple). Larger squares fill the S2 pocket to a greater extent. The bottom four panels are x, y arrays for four other cyclophilin simulations. Coloring and axes are as in the middle right panel. Note that the energy value ranges for the five x, y arrays are not identical and are as follows: NKTR (red = −7.0, purple = 5.0), PPIC (red = −5.9, purple = 1.3), PPIL2 (red = −4.7, purple = 1.9), PPWD1 (red = −7.0, purple = 1.0).
Similar articles
- Structural and Functional Insights into Human Nuclear Cyclophilins.
Rajiv C, Davis TL. Rajiv C, et al. Biomolecules. 2018 Dec 4;8(4):161. doi: 10.3390/biom8040161. Biomolecules. 2018. PMID: 30518120 Free PMC article. Review. - Structural and biological characterisation of the gut-associated cyclophilin B isoforms from Caenorhabditis elegans.
Picken NC, Eschenlauer S, Taylor P, Page AP, Walkinshaw MD. Picken NC, et al. J Mol Biol. 2002 Sep 6;322(1):15-25. doi: 10.1016/s0022-2836(02)00712-x. J Mol Biol. 2002. PMID: 12215411 - Identification of Salmonella Typhimurium Peptidyl-prolyl cis-trans Isomerase B (PPIase B) and Assessment of their Role in the Protein Folding.
Kumawat M, Karuna I, Ahlawat N, Ahlawat S. Kumawat M, et al. Protein Pept Lett. 2020;27(8):744-750. doi: 10.2174/0929866527666200225124104. Protein Pept Lett. 2020. PMID: 32096737 - Peptidyl-prolyl cis-trans isomerases, a superfamily of ubiquitous folding catalysts.
Göthel SF, Marahiel MA. Göthel SF, et al. Cell Mol Life Sci. 1999 Mar;55(3):423-36. doi: 10.1007/s000180050299. Cell Mol Life Sci. 1999. PMID: 10228556 Free PMC article. Review. - The crystal structure of human WD40 repeat-containing peptidylprolyl isomerase (PPWD1).
Davis TL, Walker JR, Ouyang H, MacKenzie F, Butler-Cole C, Newman EM, Eisenmesser EZ, Dhe-Paganon S. Davis TL, et al. FEBS J. 2008 May;275(9):2283-95. doi: 10.1111/j.1742-4658.2008.06381.x. Epub 2008 Apr 3. FEBS J. 2008. PMID: 18397323
Cited by
- Evaluating the potential of non-immunosuppressive cyclosporin analogs for targeting Toxoplasma gondii cyclophilin: Insights from structural studies.
Favretto F, Jiménez-Faraco E, Catucci G, Di Matteo A, Travaglini-Allocatelli C, Sadeghi SJ, Dominici P, Hermoso JA, Astegno A. Favretto F, et al. Protein Sci. 2024 Oct;33(10):e5157. doi: 10.1002/pro.5157. Protein Sci. 2024. PMID: 39312281 Free PMC article. - Structural basis of interaction between dimeric cyclophilin 1 and Myb1 transcription factor in Trichomonas vaginalis.
Martin T, Lou YC, Chou CC, Wei SY, Sadotra S, Cho CC, Lin MH, Tai JH, Hsu CH, Chen C. Martin T, et al. Sci Rep. 2018 Apr 3;8(1):5410. doi: 10.1038/s41598-018-23821-5. Sci Rep. 2018. PMID: 29615721 Free PMC article. - Microbial cyclophilins: specialized functions in virulence and beyond.
Dimou M, Venieraki A, Katinakis P. Dimou M, et al. World J Microbiol Biotechnol. 2017 Aug 8;33(9):164. doi: 10.1007/s11274-017-2330-6. World J Microbiol Biotechnol. 2017. PMID: 28791545 Review. - A ferroptosis-related ceRNA network in hepatocellular carcinoma for potential clinical applications.
Yang Z, He K, Chen W, Chen Y. Yang Z, et al. Am J Transl Res. 2023 Jun 15;15(6):3912-3927. eCollection 2023. Am J Transl Res. 2023. PMID: 37434825 Free PMC article. - Cyclophilin BcCyp2 Regulates Infection-Related Development to Facilitate Virulence of the Gray Mold Fungus Botrytis cinerea.
Sun J, Sun CH, Chang HW, Yang S, Liu Y, Zhang MZ, Hou J, Zhang H, Li GH, Qin QM. Sun J, et al. Int J Mol Sci. 2021 Feb 8;22(4):1694. doi: 10.3390/ijms22041694. Int J Mol Sci. 2021. PMID: 33567582 Free PMC article.
References
- Fischer G, Wittmann-Liebold B, Lang K, Kiefhaber T, Schmid F. X. Cyclophilin and peptidyl-prolyl cis-trans isomerase are probably identical proteins. Nature. 1989;337:476–478. - PubMed
- Takahashi N, Hayano T, Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473–475. - PubMed
- McKeon F. When worlds collide: immunosuppressants meet protein phosphatases. Cell. 1991;66:823–826. - PubMed
- Schreiber S. L. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials