Selective vulnerability of neurons to acute toxicity after proteasome inhibitor treatment: implications for oxidative stress and insolubility of newly synthesized proteins - PubMed (original) (raw)
Selective vulnerability of neurons to acute toxicity after proteasome inhibitor treatment: implications for oxidative stress and insolubility of newly synthesized proteins
Kalavathi Dasuri et al. Free Radic Biol Med. 2010.
Abstract
Maintaining protein homeostasis is vital to cell viability, with numerous studies demonstrating a role for proteasome inhibition occurring during the aging of a variety of tissues and, presumably, contributing to the disruption of cellular homeostasis during aging. In this study we sought to elucidate the differences between neurons and astrocytes in regard to basal levels of protein synthesis, proteasome-mediated protein degradation, and sensitivity to cytotoxicity after proteasome inhibitor treatment. In these studies we demonstrate that neurons have an increased vulnerability, compared to astrocyte cultures, to proteasome-inhibitor-induced cytotoxicity. No significant difference was observed between these two cell types in regard to the basal rates of protein synthesis, or basal rates of protein degradation, in the pool of short-lived proteins. After proteasome inhibitor treatment neuronal crude lysates were observed to undergo greater increases in the levels of ubiquitinated and oxidized proteins and selectively exhibited increased levels of newly synthesized proteins accumulating within the insoluble protein pool, compared to astrocytes. Together, these data suggest a role for increased oxidized proteins and sequestration of newly synthesized proteins in the insoluble protein pool, as potential mediators of the selective neurotoxicity after proteasome inhibitor treatment. The implications for neurons exhibiting increased sensitivity to acute proteasome inhibitor exposure, and the corresponding changes in protein homeostasis observed after proteasome inhibition, are discussed in the context of both aging and age-related disorders of the nervous system.
Copyright © 2010. Published by Elsevier Inc.
Figures
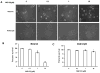
Figure 1. Proteasome inhibitors induce impairment of 20S and 26S proteasomes in both neurons and astrocytes
Primary rat astrocytes and neurons analyzed for sensitivity to proteasome inhibition following exposure to MG132. Activities of 20S and 26S were determined 1 hr following exposure to proteasome inhibitor MG132 for 1 hour, with 20S and 26S proteasome activity measured as described in methods. Data is representative of results from 2 separate experiments.
Figure 2. Proteasome inhibition induces more cell death in primary rat neurons as compared to astrocytes
Cells were treated with increasing concentrations of the proteasome inhibitor MG132 and analyzed for cell viability 24 hours post treatment. Neurons were observed to have significantly higher levels of cell death in response to proteasome inhibitor administration (A) as compared to astrocyte cultures (B). Results using morphological criteria as well as nuclear condensation/fragmentation gave nearly identical results. Data are presented as the mean and S.E.M. of results from 3 different sets of independent experiments (5 dishes per experiment).
Figure 3. The levels of oxidized proteins, but not ubiquitinated proteins, are increased to larger extent in neurons as compared to astrocytes following proteasome inhibition
Rat primary cortical neurons and astrocyte cultures were analyzed for the levels of ubiquitinated (A) and oxidized proteins (B) following treatment with proteasome inhibitors. Cells were treated with increasing concentrations of the proteasome inhibitor 10 μM MG132 and analyzed for ubiquitinated and oxidized protein levels following proteasome inhibitor treatment for 15 hours. Neurons were observed to have more severe increases in oxidized proteins following proteasome inhibitor treatment. Data are representative of results from three separate experiments.
Figure 4. The levels of short-lived protein synthesis and degradation are similar in neurons and astrocytes
Rat primary cortical neurons and astrocyte cultures were analyzed for protein synthesis following a 5 minute pulse of 35S-methionine. The levels of short lived protein degradation were analyzed in neuron and astrocyte cultures following 5 minute pulse of 35S-methionine and corresponding increasing lengths of chase period. Results indicate that neurons and astrocytes have similar levels of short lived protein synthesis (A) and short lived protein degradation (B). Data are presented as the mean and S.E.M. of results from 3 independent set of experiments with 4 dishes for each time point in an experiment.
Figure 5. Heat shock proteins in neurons and astrocytes following proteasome inhibition
Lysates from rat primary cortical neurons and astrocyte cultures were analyzed by Western blotting for the levels of heat shock protein induction following proteasome inhibition for 15 hours. Antibodies against the Hsp40, Hsp70 or Hsp90 were used in the analysis. Beta actin was used to show the equal loading of protein lysates. Data represent the 3 independent set of experiments done under similar conditions.
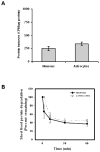
Figure 6. Proteasome inhibition resulted in increased accumulation of recently synthesized proteins in Triton x-100 insoluble fraction of neurons, as compared with astrocytes
Rat primary neurons and astrocytes were pulsed for one hour with 35S-methionine and chased for indicated time points in the presence or absence of proteasome inhibitor, MG132. Whole cell lysates were separated in to triton X-100 soluble and insoluble fractions and TCA insoluble radioactivity of these fractions was measured as described in methods. Proteasome inhibition resulted in the accumulation of higher levels of recently synthesized short lived proteins in triton X-100 insoluble fractions of primary neurons (A) when compared with the astrocytes (B). Data are presented as the mean and S.E.M. of results from 3 different sets of independent experiments.
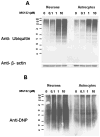
Figure 7. Proteasome inhibition resulted in increased accumulation of recently synthesized proteins, that are ubiquitinated, in triton x-100 insoluble pool of neurons as compared with astrocytes
Rat primary Neurons and astrocytes were collected after treatment with cyclohexamide for indicated time points in the presence or absence of proteasome inhibitor, MG132, as described in methods. Whole cell lysates were fractionated by Triton X-100 and the amounts of ubiquitinated proteins in Triton X-100 soluble and insoluble fractions were analyzed using western blot analysis. Results indicate the increased accumulation of recently synthesized proteins, that are higher molecular weight ubiquitinated proteins, in triton x-100 insoluble pool of neurons (A), as compared with astrocytes (B), following proteasome inhibition.
Figure 8. Primary Neuron and astrocytes showed no differences in the levels of oxidized proteins in triton x-100 soluble and insoluble fractions following proteasome inhibition
Rat primary neurons and astrocytes were collected after treatment with cyclohexamide for indicated time points in the presence or absence of proteasome inhibitor, MG132, as described in methods. Whole cell lysates were fractionated by triton X-100 and the amounts of oxidized proteins in triton X-100 soluble and insoluble fractions were analyzed as described in methods. Results did not show differences in the levels of oxidized proteins in triton x-100 insoluble and soluble fractions between neurons (A) and astrocytes (B) following proteasome inhibition.
Similar articles
- Increased protein hydrophobicity in response to aging and Alzheimer disease.
Dasuri K, Ebenezer P, Zhang L, Fernandez-Kim SO, Bruce-Keller AJ, Markesbery WR, Keller JN. Dasuri K, et al. Free Radic Biol Med. 2010 May 15;48(10):1330-7. doi: 10.1016/j.freeradbiomed.2010.02.012. Epub 2010 Feb 24. Free Radic Biol Med. 2010. PMID: 20188163 Free PMC article. - Proteasome inhibition increases DNA and RNA oxidation in astrocyte and neuron cultures.
Ding Q, Dimayuga E, Markesbery WR, Keller JN. Ding Q, et al. J Neurochem. 2004 Dec;91(5):1211-8. doi: 10.1111/j.1471-4159.2004.02802.x. J Neurochem. 2004. PMID: 15569264 - Autophagy is activated by proteasomal inhibition and involved in aggresome clearance in cultured astrocytes.
Jänen SB, Chaachouay H, Richter-Landsberg C. Jänen SB, et al. Glia. 2010 Nov 1;58(14):1766-74. doi: 10.1002/glia.21047. Glia. 2010. PMID: 20645412 - Astrocytic gap junctional communication decreases neuronal vulnerability to oxidative stress-induced disruption of Ca2+ homeostasis and cell death.
Blanc EM, Bruce-Keller AJ, Mattson MP. Blanc EM, et al. J Neurochem. 1998 Mar;70(3):958-70. doi: 10.1046/j.1471-4159.1998.70030958.x. J Neurochem. 1998. PMID: 9489715 - Degradation of oxidized proteins by the 20S proteasome.
Davies KJ. Davies KJ. Biochimie. 2001 Mar-Apr;83(3-4):301-10. doi: 10.1016/s0300-9084(01)01250-0. Biochimie. 2001. PMID: 11295490 Review.
Cited by
- Negative regulation of 26S proteasome stability via calpain-mediated cleavage of Rpn10 subunit upon mitochondrial dysfunction in neurons.
Huang Q, Wang H, Perry SW, Figueiredo-Pereira ME. Huang Q, et al. J Biol Chem. 2013 Apr 26;288(17):12161-74. doi: 10.1074/jbc.M113.464552. Epub 2013 Mar 18. J Biol Chem. 2013. PMID: 23508964 Free PMC article. - The Proteasome Inhibition Model of Parkinson's Disease.
Bentea E, Verbruggen L, Massie A. Bentea E, et al. J Parkinsons Dis. 2017;7(1):31-63. doi: 10.3233/JPD-160921. J Parkinsons Dis. 2017. PMID: 27802243 Free PMC article. Review. - NGF-Dependent Changes in Ubiquitin Homeostasis Trigger Early Cholinergic Degeneration in Cellular and Animal AD-Model.
Latina V, Caioli S, Zona C, Ciotti MT, Borreca A, Calissano P, Amadoro G. Latina V, et al. Front Cell Neurosci. 2018 Dec 13;12:487. doi: 10.3389/fncel.2018.00487. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30618634 Free PMC article. - Overexpression of Atg5 in mice activates autophagy and extends lifespan.
Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, Jung S, Jung YK. Pyo JO, et al. Nat Commun. 2013;4:2300. doi: 10.1038/ncomms3300. Nat Commun. 2013. PMID: 23939249 Free PMC article. - Walking the oxidative stress tightrope: a perspective from the naked mole-rat, the longest-living rodent.
Rodriguez KA, Wywial E, Perez VI, Lambert AJ, Edrey YH, Lewis KN, Grimes K, Lindsey ML, Brand MD, Buffenstein R. Rodriguez KA, et al. Curr Pharm Des. 2011;17(22):2290-307. doi: 10.2174/138161211797052457. Curr Pharm Des. 2011. PMID: 21736541 Free PMC article. Review.
References
- Keller JN, Hanni KB, Markesbery WR. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech Ageing Dev. 2000;113:61–70. - PubMed
- Chondrogianni N, Gonos ES. Proteasome dysfunction in mammalian aging: steps and factors involved. Exp Gerontol. 2005;40:931–938. - PubMed
- Ramanujan VK, Herman BA. Aging process modulates nonlinear dynamics in liver cell metabolism. J Biol Chem. 2007;282:19217–19226. - PubMed
- Ding Q, Dimayuga E, Keller JN. Proteasome regulation of oxidative stress in aging and age-related diseases of the CNS. Antioxid Redox Signal. 2006;8:163–172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AG025771/AG/NIA NIH HHS/United States
- R01 AG025771/AG/NIA NIH HHS/United States
- R01 AG025771-05/AG/NIA NIH HHS/United States
- AG029885/AG/NIA NIH HHS/United States
- R01 AG029885-05/AG/NIA NIH HHS/United States
- R01 AG029885/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources