Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development - PubMed (original) (raw)
Biochemical and functional interaction of disrupted-in-schizophrenia 1 and amyloid precursor protein regulates neuronal migration during mammalian cortical development
Tracy L Young-Pearse et al. J Neurosci. 2010.
Abstract
Although clinically distinct, schizophrenia and Alzheimer's disease are common and devastating disorders that profoundly impair cognitive function. For Alzheimer's disease, key mechanistic insights have emerged from genetic studies that identified causative mutations in amyloid precursor protein (APP) and presenilin. Several genes have been associated with schizophrenia and other major psychoses, and understanding their normal functions will help elucidate the underlying causes of these disorders. One such gene is disrupted-in-schizophrenia 1 (DISC1). DISC1 and APP have been implicated separately in cortical development, with each having roles in both neuronal migration and neurite outgrowth. Here, we report a previously unrecognized biochemical and functional interaction between DISC1 and APP. Using in utero electroporation in the living rat brain, we show that DISC1 acts downstream of APP and Disabled-1 to regulate cortical precursor cell migration. Specifically, overexpression of DISC1 rescues the migration defect caused by a loss of APP expression. Moreover, knockdown of APP in cultured embryonic neurons results in altered subcellular localization of DISC1. Using transfected cells and normal brain tissue, we show that APP and DISC1 coimmunoprecipitate and that the intracellular domain of APP interacts with the N-terminal domain of DISC1. Based on these findings, we hypothesize that the APP cytoplasmic region transiently interacts with DISC1 to help regulate the translocation of DISC1 to the centrosome, where it plays a key role in controlling neuronal migration during cortical development.
Figures
Figure 1.
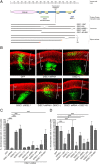
Functional domains of DISC1 involved in cortical migration. A, Schematic representation of the DISC1 gene outlining constructs used in this study. B–D, E15.5 rat cortices were coelectroporated with GFP and constructs listed. Three days later, brains were fixed, sectioned coronally, and immunostained for MAP2 (red), and the percentage of electroporated cells (green) that migrated into the cortical plate was quantified (C, D). Electroporations tested the ability of various DISC1 constructs to rescue the migration defect observed with DISC1 shRNA (C) and also the migration effects of overexpression of each DISC1 construct in the absence of DISC1 shRNA (D). Representative images for some conditions are shown (B). Scale bars, 100 μm. Data for each construct represent the average from at least three independent brains. Error bars represent SD. *p < 0.05; ***p < 0.001.
Figure 2.
DISC1 acts downstream of APP and DAB1 in cortical migration. E15.5 rat cortices were coelectroporated with GFP and the constructs listed. The APP shRNA used here is the “active” shRNA shown in supplemental Figure 1 (available at
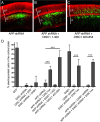
as supplemental material). Three days later, brains were fixed, sectioned coronally, and immunostained for MAP2 (red). Representative images for some conditions are shown (A–C). The percentage of electroporated cells (green) that migrated into the cortical plate was quantified (D). Scale bars, 100 μm. Data for each construct represents the average from at least three independent brains. Error bars represent SDs. ***p < 0.001.
Figure 3.
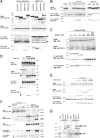
APP and DISC1 interact biochemically. COS cells were transiently transfected with constructs as listed and harvested 48 h later in 1% NP-40 lysis buffer. IPs were performed for HA using 3F10-affinity resin (A–C, E, F), or with 22C11 (anti-APP; Millipore) (D). Western blots were run of lysates and IPs as shown. In A, the asterisk (*) denotes relative sizes of each FLAG-tagged protein. In E, the asterisk (*) denotes the IgG heavy chain band resulting from the immunoprecipitation. In F, the asterisk (*) marks co-IP of endogenous APP with overexpressed DISC1-HA. G, Membrane and cytosolic preps were made from adult rat brains as described in Materials and Methods. Antibodies used for IP were anti-DISC1 (Invitrogen) and anti-APP (C9).
Figure 4.
APP knockdown at E15.5 does not affect proliferation. E15.5 rat cortices were coelectroporated with GFP and the constructs listed. Twenty-four hours later, BrdU was injected intraperitoneally, and brains were harvested 24 h after BrdU injection. Brains were fixed, sectioned coronally, and immunostained for BrdU (red) and Ki67 (blue). Representative images are shown (A–C). The percentage of electroporated cells (green) that migrated into the cortical plate (D) was quantified. The percentage of cells in the VZ/SVZ/IZ that incorporated BrdU (E) also was quantified (D). Scale bars, 50 μm. Data for each construct represent the average from at least three independent brains. Error bars represent SDs. *p < 0.05; **p < 0.01; ***p < 0.001.
Figure 5.
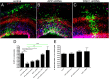
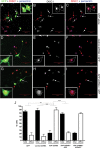
APP knockdown results in altered DISC1 localization in cortical neurons. Primary rat cortical neurons were cotransfected with GFP alone or with GFP plus control shRNA or APP shRNA constructs. Three days later, neurons were fixed and immunostained for DISC1 (Abcam) (shown in red in A, C, D, F, G, I; or white in B, E, H) and pericentrin (blue). Confocal images were acquired, and representative images are shown (A–I). The insets show magnified views of the cells indicated by red arrows. Identities of the images were deidentified, and DISC1 localization was examined in GFP-positive cotransfected cells (indicated by white arrows). Cells were classified as having focal (B, E, arrows) or diffuse DISC1 (H, arrow) immunostaining. Data from three independent experiments are shown in J. At least 50 cells were quantified for each condition. Error bars represent SDs between the three experiments. **p < 0.01; ***p < 0.001.
Figure 6.
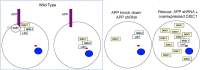
Summary of data showing APP and DISC1 interactions in cortical cell migration. In wild-type cells, APP exists in a complex with DAB1 and DISC1 through distinct binding domains in its cytoplasmic region. In addition to interacting with APP at the cell surface and/or on intracellular membranes, DISC1 localizes to the centrosome with its binding partners NDEL1 and LIS1, where it carries out its role in nucleokinesis (Morris et al., 2003; Kamiya et al., 2005; Bradshaw et al., 2008). When APP is knocked down, DISC1 is localized more diffusely throughout the cytoplasm and cannot perform its function in migration. However, when cells lacking APP are flooded with excess DISC1, the need for the APP–DISC1 biochemical interaction is bypassed and the migration defect is rescued. The small red ellipse represents the centrosome, and the blue oval represents the nucleus.
Similar articles
- Pancortins interact with amyloid precursor protein and modulate cortical cell migration.
Rice HC, Townsend M, Bai J, Suth S, Cavanaugh W, Selkoe DJ, Young-Pearse TL. Rice HC, et al. Development. 2012 Nov;139(21):3986-96. doi: 10.1242/dev.082909. Epub 2012 Sep 19. Development. 2012. PMID: 22992957 Free PMC article. - A critical function for beta-amyloid precursor protein in neuronal migration revealed by in utero RNA interference.
Young-Pearse TL, Bai J, Chang R, Zheng JB, LoTurco JJ, Selkoe DJ. Young-Pearse TL, et al. J Neurosci. 2007 Dec 26;27(52):14459-69. doi: 10.1523/JNEUROSCI.4701-07.2007. J Neurosci. 2007. PMID: 18160654 Free PMC article. - Disrupted-in-Schizophrenia-1 Attenuates Amyloid-β Generation and Cognitive Deficits in APP/PS1 Transgenic Mice by Reduction of β-Site APP-Cleaving Enzyme 1 Levels.
Deng QS, Dong XY, Wu H, Wang W, Wang ZT, Zhu JW, Liu CF, Jia WQ, Zhang Y, Schachner M, Ma QH, Xu RX. Deng QS, et al. Neuropsychopharmacology. 2016 Jan;41(2):440-53. doi: 10.1038/npp.2015.164. Epub 2015 Jun 11. Neuropsychopharmacology. 2016. PMID: 26062786 Free PMC article. - Genes and schizophrenia: beyond schizophrenia: the role of DISC1 in major mental illness.
Hennah W, Thomson P, Peltonen L, Porteous D. Hennah W, et al. Schizophr Bull. 2006 Jul;32(3):409-16. doi: 10.1093/schbul/sbj079. Epub 2006 May 12. Schizophr Bull. 2006. PMID: 16699061 Free PMC article. Review. - DISC1 in schizophrenia: genetic mouse models and human genomic imaging.
Johnstone M, Thomson PA, Hall J, McIntosh AM, Lawrie SM, Porteous DJ. Johnstone M, et al. Schizophr Bull. 2011 Jan;37(1):14-20. doi: 10.1093/schbul/sbq135. Epub 2010 Dec 13. Schizophr Bull. 2011. PMID: 21149852 Free PMC article. Review.
Cited by
- Disrupted-in-Schizophrenia 1 (DISC1) is necessary for the correct migration of cortical interneurons.
Steinecke A, Gampe C, Valkova C, Kaether C, Bolz J. Steinecke A, et al. J Neurosci. 2012 Jan 11;32(2):738-45. doi: 10.1523/JNEUROSCI.5036-11.2012. J Neurosci. 2012. PMID: 22238109 Free PMC article. - DISC1: Structure, Function, and Therapeutic Potential for Major Mental Illness.
Soares DC, Carlyle BC, Bradshaw NJ, Porteous DJ. Soares DC, et al. ACS Chem Neurosci. 2011 Nov 16;2(11):609-632. doi: 10.1021/cn200062k. Epub 2011 Aug 5. ACS Chem Neurosci. 2011. PMID: 22116789 Free PMC article. - Association between 5q23.2-located polymorphism of CTXN3 gene (Cortexin 3) and schizophrenia in European-Caucasian males; implications for the aetiology of schizophrenia.
Šerý O, Lochman J, Povová J, Janout V, Plesník J, Balcar VJ. Šerý O, et al. Behav Brain Funct. 2015 Mar 17;11:10. doi: 10.1186/s12993-015-0057-9. Behav Brain Funct. 2015. PMID: 25889058 Free PMC article. - Amyloid precursor protein and its interacting proteins in neurodevelopment.
Chau DD, Ng LL, Zhai Y, Lau KF. Chau DD, et al. Biochem Soc Trans. 2023 Aug 31;51(4):1647-1659. doi: 10.1042/BST20221527. Biochem Soc Trans. 2023. PMID: 37387352 Free PMC article. Review. - Disc1 point mutations in mice affect development of the cerebral cortex.
Lee FH, Fadel MP, Preston-Maher K, Cordes SP, Clapcote SJ, Price DJ, Roder JC, Wong AH. Lee FH, et al. J Neurosci. 2011 Mar 2;31(9):3197-206. doi: 10.1523/JNEUROSCI.4219-10.2011. J Neurosci. 2011. PMID: 21368031 Free PMC article.
References
- Bradshaw NJ, Ogawa F, Antolin-Fontes B, Chubb JE, Carlyle BC, Christie S, Claessens A, Porteous DJ, Millar JK. DISC1, PDE4B, and NDE1 at the centrosome and synapse. Biochem Biophys Res Commun. 2008;377:1091–1096. - PubMed
- Brandon NJ. Dissecting DISC1 function through protein-protein interactions. Biochem Soc Trans. 2007;35:1283–1286. - PubMed
- Brandon NJ, Handford EJ, Schurov I, Rain JC, Pelling M, Duran-Jimeniz B, Camargo LM, Oliver KR, Beher D, Shearman MS, Whiting PJ. Disrupted in schizophrenia 1 and Nudel form a neurodevelopmentally regulated protein complex: implications for schizophrenia and other major neurological disorders. Mol Cell Neurosci. 2004;25:42–55. - PubMed
- Camargo LM, Collura V, Rain JC, Mizuguchi K, Hermjakob H, Kerrien S, Bonnert TP, Whiting PJ, Brandon NJ. Disrupted in schizophrenia 1 interactome: evidence for the close connectivity of risk genes and a potential synaptic basis for schizophrenia. Mol Psychiatry. 2007;12:74–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG06173/AG/NIA NIH HHS/United States
- K99 MH85004/MH/NIMH NIH HHS/United States
- R01 AG006173/AG/NIA NIH HHS/United States
- K99 MH085004/MH/NIMH NIH HHS/United States
- R00 MH085004/MH/NIMH NIH HHS/United States
- R37 AG006173/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources