The international limits and population at risk of Plasmodium vivax transmission in 2009 - PubMed (original) (raw)
doi: 10.1371/journal.pntd.0000774.
Rosalind E Howes, Anand P Patil, Peter W Gething, Thomas P Van Boeckel, William H Temperley, Caroline W Kabaria, Andrew J Tatem, Bui H Manh, Iqbal R F Elyazar, J Kevin Baird, Robert W Snow, Simon I Hay
Affiliations
- PMID: 20689816
- PMCID: PMC2914753
- DOI: 10.1371/journal.pntd.0000774
The international limits and population at risk of Plasmodium vivax transmission in 2009
Carlos A Guerra et al. PLoS Negl Trop Dis. 2010.
Abstract
Background: A research priority for Plasmodium vivax malaria is to improve our understanding of the spatial distribution of risk and its relationship with the burden of P. vivax disease in human populations. The aim of the research outlined in this article is to provide a contemporary evidence-based map of the global spatial extent of P. vivax malaria, together with estimates of the human population at risk (PAR) of any level of transmission in 2009.
Methodology: The most recent P. vivax case-reporting data that could be obtained for all malaria endemic countries were used to classify risk into three classes: malaria free, unstable (<0.1 case per 1,000 people per annum (p.a.)) and stable (> or =0.1 case per 1,000 p.a.) P. vivax malaria transmission. Risk areas were further constrained using temperature and aridity data based upon their relationship with parasite and vector bionomics. Medical intelligence was used to refine the spatial extent of risk in specific areas where transmission was reported to be absent (e.g., large urban areas and malaria-free islands). The PAR under each level of transmission was then derived by combining the categorical risk map with a high resolution population surface adjusted to 2009. The exclusion of large Duffy negative populations in Africa from the PAR totals was achieved using independent modelling of the gene frequency of this genetic trait. It was estimated that 2.85 billion people were exposed to some risk of P. vivax transmission in 2009, with 57.1% of them living in areas of unstable transmission. The vast majority (2.59 billion, 91.0%) were located in Central and South East (CSE) Asia, whilst the remainder were located in America (0.16 billion, 5.5%) and in the Africa+ region (0.10 billion, 3.5%). Despite evidence of ubiquitous risk of P. vivax infection in Africa, the very high prevalence of Duffy negativity throughout Central and West Africa reduced the PAR estimates substantially.
Conclusions: After more than a century of development and control, P. vivax remains more widely distributed than P. falciparum and is a potential cause of morbidity and mortality amongst the 2.85 billion people living at risk of infection, the majority of whom are in the tropical belt of CSE Asia. The probability of infection is reduced massively across Africa by the frequency of the Duffy negative trait, but transmission does occur on the continent and is a concern for Duffy positive locals and travellers. The final map provides the spatial limits on which the endemicity of P. vivax transmission can be mapped to support future cartographic-based burden estimations.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
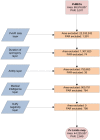
Figure 1. Flow chart of the various data and exclusion layers used to derive the final map.
The pink rectangle denotes the surface area and populations of _Pv_MECs, whilst the pink ovoid represents the resulting trimmed surface area and PAR after the exclusion of risk by the various input layers, denoted by the blue rhomboids. Orange rectangles show area and PAR exclusions at each step to illustrate how these were reduced progressively. The sequence in which the exclusion layers are applied does not affect the final PAR estimates.
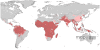
Figure 2. Plasmodium vivax malaria risk defined by _Pv_API data.
Transmission was defined as stable (red areas, where _Pv_API≥0.1 per 1,000 people p.a.), unstable (pink areas, where _Pv_API<0.1 per 1,000 p.a.) or no risk (grey areas). The boundaries of the 95 countries defined as P. vivax endemic are shown.
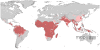
Figure 3. Further refinement of Plasmodium vivax transmission risk areas using the temperature layer of exclusion.
Risk areas are defined as in Figure 2.
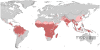
Figure 4. Aridity layer overlaid on the _Pv_API and temperature layers.
Risk areas are defined as in Figure 2.
Figure 5. The global spatial limits of Plasmodium vivax malaria transmission in 2009.
Risk areas are defined as in Figure 2. The medical intelligence and predicted Duffy negativity layers are overlaid on the P. vivax limits of transmission as defined by the _Pv_API data and biological mask layers. Areas where Duffy negativity prevalence was estimated as ≥90% are hatched, indicating where PAR estimates were modulated most significantly by the presence of this genetic trait.
Similar articles
- A world malaria map: Plasmodium falciparum endemicity in 2007.
Hay SI, Guerra CA, Gething PW, Patil AP, Tatem AJ, Noor AM, Kabaria CW, Manh BH, Elyazar IR, Brooker S, Smith DL, Moyeed RA, Snow RW. Hay SI, et al. PLoS Med. 2009 Mar 24;6(3):e1000048. doi: 10.1371/journal.pmed.1000048. PLoS Med. 2009. PMID: 19323591 Free PMC article. - A long neglected world malaria map: Plasmodium vivax endemicity in 2010.
Gething PW, Elyazar IR, Moyes CL, Smith DL, Battle KE, Guerra CA, Patil AP, Tatem AJ, Howes RE, Myers MF, George DB, Horby P, Wertheim HF, Price RN, Müeller I, Baird JK, Hay SI. Gething PW, et al. PLoS Negl Trop Dis. 2012;6(9):e1814. doi: 10.1371/journal.pntd.0001814. Epub 2012 Sep 6. PLoS Negl Trop Dis. 2012. PMID: 22970336 Free PMC article. - Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.
Crider K, Williams J, Qi YP, Gutman J, Yeung L, Mai C, Finkelstain J, Mehta S, Pons-Duran C, Menéndez C, Moraleda C, Rogers L, Daniels K, Green P. Crider K, et al. Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article. - Defining the global spatial limits of malaria transmission in 2005.
Guerra CA, Snow RW, Hay SI. Guerra CA, et al. Adv Parasitol. 2006;62:157-79. doi: 10.1016/S0065-308X(05)62005-2. Adv Parasitol. 2006. PMID: 16647970 Free PMC article. Review. - The global public health significance of Plasmodium vivax.
Battle KE, Gething PW, Elyazar IR, Moyes CL, Sinka ME, Howes RE, Guerra CA, Price RN, Baird KJ, Hay SI. Battle KE, et al. Adv Parasitol. 2012;80:1-111. doi: 10.1016/B978-0-12-397900-1.00001-3. Adv Parasitol. 2012. PMID: 23199486 Review.
Cited by
- Severe morbidity and mortality risk from malaria in the United States, 1985-2011.
Hwang J, Cullen KA, Kachur SP, Arguin PM, Baird JK. Hwang J, et al. Open Forum Infect Dis. 2014 Jun 30;1(1):ofu034. doi: 10.1093/ofid/ofu034. eCollection 2014 Mar. Open Forum Infect Dis. 2014. PMID: 25734104 Free PMC article. - Fy(a)/Fy(b) antigen polymorphism in human erythrocyte Duffy antigen affects susceptibility to Plasmodium vivax malaria.
King CL, Adams JH, Xianli J, Grimberg BT, McHenry AM, Greenberg LJ, Siddiqui A, Howes RE, da Silva-Nunes M, Ferreira MU, Zimmerman PA. King CL, et al. Proc Natl Acad Sci U S A. 2011 Dec 13;108(50):20113-8. doi: 10.1073/pnas.1109621108. Epub 2011 Nov 28. Proc Natl Acad Sci U S A. 2011. PMID: 22123959 Free PMC article. - Evaluation of Chimpanzee Adenovirus and MVA Expressing TRAP and CSP from Plasmodium cynomolgi to Prevent Malaria Relapse in Nonhuman Primates.
Kim YC, Dema B, Rodriguez-Garcia R, López-Camacho C, Leoratti FMS, Lall A, Remarque EJ, Kocken CHM, Reyes-Sandoval A. Kim YC, et al. Vaccines (Basel). 2020 Jul 6;8(3):363. doi: 10.3390/vaccines8030363. Vaccines (Basel). 2020. PMID: 32640702 Free PMC article. - Sources of spatial animal and human health data: Casting the net wide to deal more effectively with increasingly complex disease problems.
Stevens KB, Pfeiffer DU. Stevens KB, et al. Spat Spatiotemporal Epidemiol. 2015 Apr;13:15-29. doi: 10.1016/j.sste.2015.04.003. Epub 2015 May 8. Spat Spatiotemporal Epidemiol. 2015. PMID: 26046634 Free PMC article. Review. - Population structure and transmission dynamics of Plasmodium vivax in the Republic of Korea based on microsatellite DNA analysis.
Iwagami M, Fukumoto M, Hwang SY, Kim SH, Kho WG, Kano S. Iwagami M, et al. PLoS Negl Trop Dis. 2012;6(4):e1592. doi: 10.1371/journal.pntd.0001592. Epub 2012 Apr 3. PLoS Negl Trop Dis. 2012. PMID: 22509416 Free PMC article.
References
- Snow RW, Craig MH, Newton CRJC, Steketee RW. The public health burden of Plasmodium falciparum malaria in Africa: deriving the numbers. Working Paper No. 11. Bethesda, Maryland, U.S.A.: Disease Control Priorities Project, Fogarty International Center, National Institutes of Health; 2003.
- Mendis K, Sina BJ, Marchesini P, Carter R. The neglected burden of Plasmodium vivax malaria. Am J Trop Med Hyg. 2001;64:97–106. - PubMed
- Baird JK. Neglect of Plasmodium vivax malaria. Trends Parasitol. 2007;23:533–539. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources