Assembly of fibronectin extracellular matrix - PubMed (original) (raw)
Review
Assembly of fibronectin extracellular matrix
Purva Singh et al. Annu Rev Cell Dev Biol. 2010.
Abstract
In the process of matrix assembly, multivalent extracellular matrix (ECM) proteins are induced to self-associate and to interact with other ECM proteins to form fibrillar networks. Matrix assembly is usually initiated by ECM glycoproteins binding to cell surface receptors, such as fibronectin (FN) dimers binding to α5ß1 integrin. Receptor binding stimulates FN self-association mediated by the N-terminal assembly domain and organizes the actin cytoskeleton to promote cell contractility. FN conformational changes expose additional binding sites that participate in fibril formation and in conversion of fibrils into a stabilized, insoluble form. Once assembled, the FN matrix impacts tissue organization by contributing to the assembly of other ECM proteins. Here, we describe the major steps, molecular interactions, and cellular mechanisms involved in assembling FN dimers into fibrillar matrix while highlighting important issues and major questions that require further investigation.
Figures
Figure 1
Diagram of a fibronectin (FN) subunit. Each FN subunit consists of three types of repeats: type I (hexagon), type II (square) and type III (cylinder). Based on rotary shadowing electron microscopy images, the two subunits of FN are curved with a contour similar to the shape in this diagram (Engel et al. 1981). Domains required to initiate assembly (red ) include the cell-binding domain (RGD site in III10 + synergy site in III9), the N-terminal assembly domain (I1–5), and the intermolecular dimer cysteines at the C terminus. The 70-kDa fragment extends from I1 through I9, including the assembly and the collagen/gelatin binding domains. The III1–2 domain (red with stripes) has two FN-binding sites and participates in conformational changes that promote assembly. Other FN-binding sites are located in the III4–5 domain and in the III12–14/hepII domain that also binds to heparin and syndecans. Alternatively spliced extra domains EIIIA, EIIIB, and the variable region (V) are shown in white.
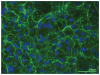
Figure 2
Fibronectin (FN) fibrillar matrix surrounds cells in culture. HT1080 cells were grown on a glass coverslip for 20 hours in medium supplemented with 0.1 μM dexamethasone and 25 μg ml−1 rat plasma FN as described in Brenner et al. (2000). Cells were fixed and stained with anti-FN monoclonal antibody (IC3) followed by fluorescein-tagged goat antimouse immunoglobulin G. Image shows FN fibrils ( green) around cells with 4′,6-diamidino-2-phenylindole (DAPI)-stained nuclei (blue).
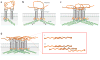
Figure 3
Major steps in fibronectin (FN) matrix assembly. Integrin-induced conversion of compact FN to extended fibrils is shown in four steps. (a) A compact FN dimer binds to integrins ( gray). FN subunits of a single dimer are shown in two shades of orange. (b) Intracellular proteins ( pink, yellow, blue) are recruited to integrin cytoplasmic domains and connected to the actin cytoskeleton ( green). Cytoskeletal connections increase cell contractility (arrows), which induces conformational changes in FN. (c) Integrin clustering and exposed FN-binding sites promote FN-FN interactions and further changes in FN conformation. (d ) Finally, these events trigger formation of stable insoluble fibrillar matrix. The inset (red box) shows interactions between single subunits of FN dimers. N indicates the N terminus of an FN subunit. Fibrils form through (i) end-to-end association of FN dimers, mediated by the N -terminal assembly domain, followed by (ii) lateral associations between fibrils that are likely to involve the other FN-binding sites in III1–2, III4–5, and III12–14. Gray X’s represent interactions between fibrils.
Figure 4
Migration of HT1080 cells on fibrillar fibronectin (FN) matrix. Extraction of a highly confluent fibroblast culture was used to prepare a cell-free fibrillar matrix in which FN is the major protein component. HT1080 human fibrosarcoma cells were allowed to attach to the matrix in serum-free medium for 2 h. Fetal bovine serum was then added to the medium to a final concentration of 10% to initiate migration. To watch the migration in action, please see Supplemental Video 1, which was originally published in Mao & Schwarzbauer (2006).
Figure 5
Future issues. The basic steps of fibronectin (FN) matrix assembly are boxed in green. Other proteins, interactions, processes, and mechanisms (blue) might impact various steps in matrix assembly as indicated (dashed blue arrows). Additional investigation is required to understand their effects. ECM, extracellular matrix.
Similar articles
- The ins and outs of fibronectin matrix assembly.
Wierzbicka-Patynowski I, Schwarzbauer JE. Wierzbicka-Patynowski I, et al. J Cell Sci. 2003 Aug 15;116(Pt 16):3269-76. doi: 10.1242/jcs.00670. J Cell Sci. 2003. PMID: 12857786 Review. - Fibronectin: Molecular Structure, Fibrillar Structure and Mechanochemical Signaling.
Dalton CJ, Lemmon CA. Dalton CJ, et al. Cells. 2021 Sep 16;10(9):2443. doi: 10.3390/cells10092443. Cells. 2021. PMID: 34572092 Free PMC article. Review. - The role of integrin binding sites in fibronectin matrix assembly in vivo.
Leiss M, Beckmann K, Girós A, Costell M, Fässler R. Leiss M, et al. Curr Opin Cell Biol. 2008 Oct;20(5):502-7. doi: 10.1016/j.ceb.2008.06.001. Epub 2008 Jul 21. Curr Opin Cell Biol. 2008. PMID: 18586094 Review. - Roles of integrins in fibronectin matrix assembly.
Wu C. Wu C. Histol Histopathol. 1997 Jan;12(1):233-40. Histol Histopathol. 1997. PMID: 9046058 Review. - Fibronectin fibril alignment is established upon initiation of extracellular matrix assembly.
Garrison CM, Schwarzbauer JE. Garrison CM, et al. Mol Biol Cell. 2021 Apr 15;32(8):739-752. doi: 10.1091/mbc.E20-08-0533. Epub 2021 Feb 24. Mol Biol Cell. 2021. PMID: 33625865 Free PMC article.
Cited by
- Defining the extracellular matrix using proteomics.
Byron A, Humphries JD, Humphries MJ. Byron A, et al. Int J Exp Pathol. 2013 Apr;94(2):75-92. doi: 10.1111/iep.12011. Epub 2013 Feb 19. Int J Exp Pathol. 2013. PMID: 23419153 Free PMC article. Review. - Periostin and matrix stiffness combine to regulate myofibroblast differentiation and fibronectin synthesis during palatal healing.
Nikoloudaki G, Snider P, Simmons O, Conway SJ, Hamilton DW. Nikoloudaki G, et al. Matrix Biol. 2020 Dec;94:31-56. doi: 10.1016/j.matbio.2020.07.002. Epub 2020 Aug 7. Matrix Biol. 2020. PMID: 32777343 Free PMC article. - Selective Induction of Molecular Assembly to Tissue-Level Anisotropy on Peptide-Based Optoelectronic Cardiac Biointerfaces.
Yao ZF, Kuang Y, Wu HT, Lundqvist E, Fu X, Celt N, Pei J, Yee AF, Ardoña HAM. Yao ZF, et al. Adv Mater. 2024 May;36(21):e2312231. doi: 10.1002/adma.202312231. Epub 2024 Feb 19. Adv Mater. 2024. PMID: 38335948 - Myeloperoxidase-derived damage to human plasma fibronectin: Modulation by protein binding and thiocyanate ions (SCN-).
Vanichkitrungruang S, Chuang CY, Hawkins CL, Davies MJ. Vanichkitrungruang S, et al. Redox Biol. 2020 Sep;36:101641. doi: 10.1016/j.redox.2020.101641. Epub 2020 Jul 9. Redox Biol. 2020. PMID: 32863239 Free PMC article. - Directional cell movement through tissues is controlled by exosome secretion.
Sung BH, Ketova T, Hoshino D, Zijlstra A, Weaver AM. Sung BH, et al. Nat Commun. 2015 May 13;6:7164. doi: 10.1038/ncomms8164. Nat Commun. 2015. PMID: 25968605 Free PMC article.
References
- Aguirre KM, McCormick RJ, Schwarzbauer JE. Fibronectin self-association is mediated by complementary sites within the amino-terminal one-third of the molecule. J Biol Chem. 1994;269:27863–68. - PubMed
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. New York: Garland Science; 2008. p. 1268.
- Anliker B, Chun J. Cell surface receptors in lysophospholipid signaling. Semin Cell Dev Biol. 2004;15:457–65. - PubMed
- Aota S, Nomizu M, Yamada K. The short amino acid sequence Pro-His-Ser-Arg-Asn in human fibronectin enhances cell adhesive function. J Biol Chem. 1994;269:24756–61. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous