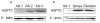HSP70: a promising target for laryngeal carcinoma radiaotherapy by inhibiting cleavage and degradation of nucleolin - PubMed (original) (raw)
HSP70: a promising target for laryngeal carcinoma radiaotherapy by inhibiting cleavage and degradation of nucleolin
Jing Xu et al. J Exp Clin Cancer Res. 2010.
Abstract
Previous studies have shown that heat shock proteins (HSPs) were upregulated in various types of tumors and were associated with histological grade, recurrence and metastasis of malignant tumors. In this study, we investigated whether heat shock protein 70 kDa (HSP70) was associated with histological grade of laryngeal squamous cell carcinomas (LSCC). We also determine the role of HSP70 in LSCC radiation resistance using a laryngeal carcinoma xenograft model by antisense HSP70 RNA technique. Immunohistochemistry data showed that HSP70 was detected in 96% of LSCC tissues (48 out of 50). The expression level of HSP70 was significantly lower in early stage of LSCC than that in late stage (P = 0.015). Radiation treatment result showed that the volumes and weights of implantation tumors in the group injected with antisense HSP70 oligos were significantly reduced comparing to the group injected with random oligos(p < 0.05). In addition, cleavage and degradation of tumor nucleolin in antisense HSP70 oligos injection group was significantly higher than that in random oligos injection group. Our result suggested that HSP70 may play a role in LSCC radiotherapy resistance by inhibiting cleavage and degradation of nucleolin.
Figures
Figure 1
HSP70 expression in LSCC tissues. High HSP70 expression in late stage of LSCC tissues (a-b) was showed comparing to the low HSP70 expression in early stage LSCC(c), (d) is the negative control show the specificity of the antibody. HSP70 shows strong expression (scored as 3+, a), moderate expression (scored as 2+, b), weak expression (scored as 1+, c), and negative expression (scored as 0, d) in cytoplasm(×400).
Figure 2
Knock-down effect of HSP70 antisense oligos. Hep-2 cells were transfected with HSP70 antisense oligos (AS) or control oligos (sense oligos and random oligos) as described under materials and methods. After incubation for 48 h, cells were harvested, lysed. Western-blotted (WB) with the corresponding antibodies was carried out to show the knock-down effect of AS. AS-1 has the best knock-down effect of all 3 oligos.
Figure 3
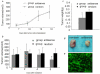
Effect of HSP70 antisense oligos on radiotherapy of laryngeal carcinoma xenografts. (a) shows the tumor growth curve before radiation, no difference between the 2 groups were found, the tumor volum were for antisense and random group, respectively (P > 0.05, 368 ± 129 mm3vs 384 ± 179 mm3). (b)There was significant difference in tumor volumes after 5Gy radiation (P < 0.05, 229 ± 28 mm3 vs 417 ± 103 mm3) (c) Tumor weights also showed significant difference after 5Gy radiation (P < 0.05, 0.18 ± 0.04 g vs 0.27 ± 0.05 g). (d) showed the representative sample of group antisense and group random after 5Gy radiation (e) showed the infection efficiency of intratumoral injection.:100×. n = 8 per group,* < 0.05.
Figure 4
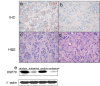
HSP70 expressions in laryngeal carcinoma xenograft were down-regulated by HSP70 antisense oligos. (a) shows HSP70 expression in implantation tumor treated with random oligos. (b) shows HSP70 expression in implantation tumor treated with HSP70 antisense oligos. (c-d) shows the representative H&E images in group random negative controls and group antisense; Western blot shows hsp70 expressions in group antisense and group random (e). HSP70 expression is significantly reduced in the antisense group comparing with random group.
Figure 5
Expression levels of HSP70 and cleavage and down-regulation of C23. (a) Western blot detected HSP70 and C23 expression in group antisense and group random; (b-c) the representative images of TUNEL assay in group antisense and group random; (d-e) The representative H&E images in group antisense and group random negative controls (×400).
Similar articles
- Advances in the study of HSP70 inhibitors to enhance the sensitivity of tumor cells to radiotherapy.
Du S, Liu Y, Yuan Y, Wang Y, Chen Y, Wang S, Chi Y. Du S, et al. Front Cell Dev Biol. 2022 Aug 10;10:942828. doi: 10.3389/fcell.2022.942828. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36036010 Free PMC article. Review. - Heat shock protein 70 inhibits hydrogen peroxide-induced nucleolar fragmentation via suppressing cleavage and down-regulation of nucleolin.
Wang K, Deng G, Chen G, Liu M, Yi Y, Yang T, McMillan DR, Xiao X. Wang K, et al. Cell Stress Chaperones. 2012 Jan;17(1):121-30. doi: 10.1007/s12192-011-0292-4. Epub 2011 Sep 30. Cell Stress Chaperones. 2012. PMID: 21960124 Free PMC article. - circPARD3 drives malignant progression and chemoresistance of laryngeal squamous cell carcinoma by inhibiting autophagy through the PRKCI-Akt-mTOR pathway.
Gao W, Guo H, Niu M, Zheng X, Zhang Y, Xue X, Bo Y, Guan X, Li Z, Guo Y, He L, Zhang Y, Li L, Cao J, Wu Y. Gao W, et al. Mol Cancer. 2020 Nov 24;19(1):166. doi: 10.1186/s12943-020-01279-2. Mol Cancer. 2020. PMID: 33234130 Free PMC article. - Down-regulation of MiR-206 promotes proliferation and invasion of laryngeal cancer by regulating VEGF expression.
Zhang T, Liu M, Wang C, Lin C, Sun Y, Jin D. Zhang T, et al. Anticancer Res. 2011 Nov;31(11):3859-63. Anticancer Res. 2011. PMID: 22110210 - Antisense-mediated downregulation of anti-apoptotic proteins induces apoptosis and sensitizes head and neck squamous cell carcinoma cells to chemotherapy.
Sharma H, Sen S, Lo Muzio L, Mariggiò A, Singh N. Sharma H, et al. Cancer Biol Ther. 2005 Jul;4(7):720-7. doi: 10.4161/cbt.4.7.1783. Epub 2005 Jul 2. Cancer Biol Ther. 2005. PMID: 15917659
Cited by
- HSP70 Family in Cancer: Signaling Mechanisms and Therapeutic Advances.
Zhao K, Zhou G, Liu Y, Zhang J, Chen Y, Liu L, Zhang G. Zhao K, et al. Biomolecules. 2023 Mar 27;13(4):601. doi: 10.3390/biom13040601. Biomolecules. 2023. PMID: 37189349 Free PMC article. Review. - Biomarkers in Laryngeal Squamous Cell Carcinoma: The Literature Review.
Verro B, Saraniti C, Carlisi D, Chiesa-Estomba C, Maniaci A, Lechien JR, Mayo M, Fakhry N, Lauricella M. Verro B, et al. Cancers (Basel). 2023 Oct 22;15(20):5096. doi: 10.3390/cancers15205096. Cancers (Basel). 2023. PMID: 37894464 Free PMC article. Review. - Advances in the study of HSP70 inhibitors to enhance the sensitivity of tumor cells to radiotherapy.
Du S, Liu Y, Yuan Y, Wang Y, Chen Y, Wang S, Chi Y. Du S, et al. Front Cell Dev Biol. 2022 Aug 10;10:942828. doi: 10.3389/fcell.2022.942828. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36036010 Free PMC article. Review. - RNA-binding protein nucleolin in disease.
Abdelmohsen K, Gorospe M. Abdelmohsen K, et al. RNA Biol. 2012 Jun;9(6):799-808. doi: 10.4161/rna.19718. Epub 2012 May 23. RNA Biol. 2012. PMID: 22617883 Free PMC article. Review. - The anti-tumor diterpene oridonin is a direct inhibitor of Nucleolin in cancer cells.
Vasaturo M, Cotugno R, Fiengo L, Vinegoni C, Dal Piaz F, De Tommasi N. Vasaturo M, et al. Sci Rep. 2018 Nov 13;8(1):16735. doi: 10.1038/s41598-018-35088-x. Sci Rep. 2018. PMID: 30425290 Free PMC article.
References
- Didelot C, Schmitt E, Brunet M, Maingret L, Parcellier A, Garrido C. Heat shock proteins: endogenous modulators of apoptotic cell death. Handb Exp Pharmacol. 2006. pp. 171–198. full_text. - PubMed
- Koga F, Tsutsumi S, Neckers LM. Low dose geldanamycin inhibits hepatocyte growth factor and hypoxia-stimulated invasion of cancer cells. Cell Cycle. 2007;6:1393–1402. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources