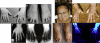Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems - PubMed (original) (raw)
Case Reports
Haploinsufficiency of HDAC4 causes brachydactyly mental retardation syndrome, with brachydactyly type E, developmental delays, and behavioral problems
Stephen R Williams et al. Am J Hum Genet. 2010.
Abstract
Brachydactyly mental retardation syndrome (BDMR) is associated with a deletion involving chromosome 2q37. BDMR presents with a range of features, including intellectual disabilities, developmental delays, behavioral abnormalities, sleep disturbance, craniofacial and skeletal abnormalities (including brachydactyly type E), and autism spectrum disorder. To date, only large deletions of 2q37 have been reported, making delineation of a critical region and subsequent identification of candidate genes difficult. We present clinical and molecular analysis of six individuals with overlapping deletions involving 2q37.3 that refine the critical region, reducing the candidate genes from >20 to a single gene, histone deacetylase 4 (HDAC4). Driven by the distinct hand and foot anomalies and similar cognitive features, we identified other cases with clinical findings consistent with BDMR but without a 2q37 deletion, and sequencing of HDAC4 identified de novo mutations, including one intragenic deletion probably disrupting normal splicing and one intragenic insertion that results in a frameshift and premature stop codon. HDAC4 is a histone deacetylase that regulates genes important in bone, muscle, neurological, and cardiac development. Reportedly, Hdac4(-/-) mice have severe bone malformations resulting from premature ossification of developing bones. Data presented here show that deletion or mutation of HDAC4 results in reduced expression of RAI1, which causes Smith-Magenis syndrome when haploinsufficient, providing a link to the overlapping findings in these disorders. Considering the known molecular function of HDAC4 and the mouse knockout phenotype, taken together with deletion or mutation of HDAC4 in multiple subjects with BDMR, we conclude that haploinsufficiency of HDAC4 results in brachydactyly mental retardation syndrome.
Figures
Figure 1
Delineation of the BDMR Critical Region (A) Ideogram of chromosome 2 band q37.3. (B) Schematic representation of 2q37.3 region and RefSeq Genes included. (C) Horizontal bars indicate the regions of deletion in each of these key 2q37 deletion syndrome cases. The brachydactyly mental retardation syndrome critical region is indicated by the vertical bars. The SMS117 HDAC4 point mutation is indicated by the small vertical line. The SMS245 HDAC4 intronic deletion is indicated by the small vertical line. (D) Quantitative real-time PCR of mRNA from lymphoblastoid cell lines from subject 2232 (del2q37.3 including HDAC4) and subject 2282 (del2q37.3 not including HDAC4) relative to control subject (no HDAC4 mutation or deletion). Assay performed twice in triplicate. Bars represent standard deviation from the mean. Data show ∼50% reduction in HDAC4 expression in 2232 and no change in 2282.
Figure 2
Skeletal Anomalies Observed in BDMR (A) Hands of case SMS117. (B) Feet of SMS117, showing shortened 4th metatarsal and wide spacing between toes. (C) Radiograph of left hand of SMS117 showing shortened 3rd, 4th, and 5th metacarpals. (D) Radiograph of SMS117 feet showing proximally placed and shortened 4th metatarsal and bilaterally widely spaced 1st, 2nd, and 3rd toes. (E) Photo of SMS272 at 17 years of age. (F) Hands of SMS272. Note shortened 3rd and 4th fingers. (G) Feet of SMS272 with shortened 3rd toes and proximally placed and shortened 4th toes. (H) Radiograph documenting shortened 3rd and 4th metacarpals in the hands of SMS272.
Figure 3
SMS117 HDAC4 Mutation and Impact on HDAC4 Protein (A) SMS117 at 13 years. (B) Electropherogram trace showing nucleotide insertion of a single cytosine at c.2399 in the HDAC4 gene. (C) Partial amino acid alignment of the normal HDAC4 protein with the altered protein probably produced in SMS117 resulting from frameshift mutation. Premature stop codon highlighted in yellow. The histone deacetylase domain resides at amino acids 655–1084 (blue line) and the nuclear export signal is at amino acids 1051–1084 (orange line). The mutation disrupts the HDAC domain and removes the nuclear export signal.
Figure 4
SMS245 HDAC4 Mutation (A) SMS245, age 10. (B) Electropherogram trace showing deletion start of SMS245 HDAC4 intron 5 at bp Chr2:239,763,222 (c.490+56_121del65). (C) Alignment of SMS245 normal allele with SMS245 deleted allele. Note 65 bp intronic deletion. Performed with ClustalW accessory application in BioEdit sequence alignment editor.
Figure 5
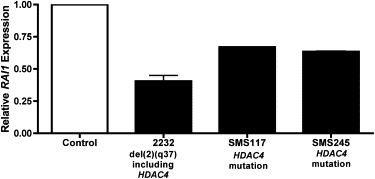
RAI1 Expression Is Reduced in HDAC4 Deletion and Mutation Cases Quantitative real-time PCR of mRNA from lymphoblastoid cells from subject 2232 (del2q37.3) and SMS117 (HDAC4 mutation) or lymphocytes from whole blood (SMS245, HDAC4 mutation) relative to control subjects (no HDAC4 mutation or deletion), as described in Material and Methods. Assay performed twice in triplicate. Bars represent standard deviation from the mean. Control RNA for real-time PCR assays came from “like” samples, i.e., blood or cultured cells.
Similar articles
- Phenotypic variant of Brachydactyly-mental retardation syndrome in a family with an inherited interstitial 2q37.3 microdeletion including HDAC4.
Villavicencio-Lorini P, Klopocki E, Trimborn M, Koll R, Mundlos S, Horn D. Villavicencio-Lorini P, et al. Eur J Hum Genet. 2013 Jul;21(7):743-8. doi: 10.1038/ejhg.2012.240. Epub 2012 Nov 28. Eur J Hum Genet. 2013. PMID: 23188045 Free PMC article. - Genotype and phenotype correlation in 103 individuals with 2q37 deletion syndrome reveals incomplete penetrance and supports HDAC4 as the primary genetic contributor.
Le TN, Williams SR, Alaimo JT, Elsea SH. Le TN, et al. Am J Med Genet A. 2019 May;179(5):782-791. doi: 10.1002/ajmg.a.61089. Epub 2019 Mar 7. Am J Med Genet A. 2019. PMID: 30848064 Review. - Haploinsufficiency of HDAC4 does not cause intellectual disability in all affected individuals.
Wheeler PG, Huang D, Dai Z. Wheeler PG, et al. Am J Med Genet A. 2014 Jul;164A(7):1826-9. doi: 10.1002/ajmg.a.36542. Epub 2014 Apr 8. Am J Med Genet A. 2014. PMID: 24715439 - Dose dependent expression of HDAC4 causes variable expressivity in a novel inherited case of brachydactyly mental retardation syndrome.
Morris B, Etoubleau C, Bourthoumieu S, Reynaud-Perrine S, Laroche C, Lebbar A, Yardin C, Elsea SH. Morris B, et al. Am J Med Genet A. 2012 Aug;158A(8):2015-20. doi: 10.1002/ajmg.a.35463. Epub 2012 Jun 29. Am J Med Genet A. 2012. PMID: 22753018 - 2q37 Microdeletion Syndrome – RETIRED CHAPTER, FOR HISTORICAL REFERENCE ONLY.
Doherty ES, Lacbawan FL. Doherty ES, et al. 2007 May 3 [updated 2013 Jan 31]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. 2007 May 3 [updated 2013 Jan 31]. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2025. PMID: 20301337 Free Books & Documents. Review.
Cited by
- Increased Abundance of Nuclear HDAC4 Impairs Neuronal Development and Long-Term Memory.
Main P, Tan WJ, Wheeler D, Fitzsimons HL. Main P, et al. Front Mol Neurosci. 2021 Mar 30;14:616642. doi: 10.3389/fnmol.2021.616642. eCollection 2021. Front Mol Neurosci. 2021. PMID: 33859551 Free PMC article. - Deficiency of the chromatin regulator BRPF1 causes abnormal brain development.
You L, Zou J, Zhao H, Bertos NR, Park M, Wang E, Yang XJ. You L, et al. J Biol Chem. 2015 Mar 13;290(11):7114-29. doi: 10.1074/jbc.M114.635250. Epub 2015 Jan 7. J Biol Chem. 2015. PMID: 25568313 Free PMC article. - Genetic variation in the epigenetic machinery and mental health.
Murgatroyd C, Spengler D. Murgatroyd C, et al. Curr Psychiatry Rep. 2012 Apr;14(2):138-49. doi: 10.1007/s11920-012-0255-1. Curr Psychiatry Rep. 2012. PMID: 22307409 Review. - High-throughput autoantibody screening identifies differentially abundant autoantibodies in autism spectrum disorder.
Mesleh A, Ehtewish H, Lennard K, Abdesselem HB, Al-Shaban F, Decock J, Alajez NM, Arredouani A, Emara MM, Albagha O, Stanton LW, Abdulla SA, Blackburnand JM, El-Agnaf OMA. Mesleh A, et al. Front Mol Neurosci. 2023 Oct 16;16:1222506. doi: 10.3389/fnmol.2023.1222506. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37908488 Free PMC article.
References
- Falk R.E., Casas K.A. Chromosome 2q37 deletion: Clinical and molecular aspects. Am. J. Med. Genet. C. Semin. Med. Genet. 2007;145C:357–371. - PubMed
- Phelan M.C., Rogers R.C., Clarkson K.B., Bowyer F.P., Levine M.A., Estabrooks L.L., Severson M.C., Dobyns W.B. Albright hereditary osteodystrophy and del(2) (q37.3) in four unrelated individuals. Am. J. Med. Genet. 1995;58:1–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous