Human prion strain selection in transgenic mice - PubMed (original) (raw)
Human prion strain selection in transgenic mice
Kurt Giles et al. Ann Neurol. 2010 Aug.
Abstract
Objective: Transgenic (Tg) mice expressing chimeras of mouse and human prion proteins (PrPs) have shorter incubation periods for Creutzfeldt-Jakob disease (CJD) prions than mice expressing full-length human PrP. Increasing the sequence similarity of the chimeric PrP to mouse PrP, by reverting human residues to mouse, resulted in a Tg line, denoted Tg22372, which was susceptible to sporadic (s) CJD prions in approximately 110 days.
Methods: Mice expressing chimeric mouse/human PrP transgenes were produced. The mice were inoculated intracerebrally with extracts prepared from the brains of patients who died of CJD. Onset of neurological dysfunction marked the end of the incubation time. After sacrifice of the Tg mice, their brains were analyzed for PrP(Sc) and neuropathological changes.
Results: Reversion of 1 additional residue (M111V) resulted in a new Tg line, termed Tg1014, susceptible to sCJD prions in approximately 75 days. Tg1014 mice also have shorter incubation periods for variant (v) CJD prions, providing a more tractable model for studying this prion strain. Transmission of vCJD prions to Tg1014 mice resulted in 2 different strains, determined by neuropathology and biochemical analysis, which correlated with the length of the incubation time. One strain had the biochemical, neuropathological, and transmission characteristics, including longer incubation times, of the inoculated vCJD strain; the second strain produced a phenotype resembling that of sCJD prions including relatively shorter incubation periods. Mice with intermediate incubation periods for vCJD prions had a mixture of the 2 strains. Both strains were serially transmitted in Tg1014 mice, which led to further reduction in incubation periods. Conversion of vCJD-like to sCJD-like strains was favored in Tg1014 mice more than in the Tg22372 line. The single amino acid difference therefore appears to offer selective pressure for propagation of the sCJD-like strain.
Interpretation: These 2 Tg mouse lines provide relatively rapid models to study human prion diseases as well as the evolution of human prion strains.
Figures
Fig. 1
Western blotting of brain homogenates after limited digestion with PK shows strain typing of sCJD (A, B) and vCJD (C, D) prions. (A, B) sCJD prions in the brain of human sCJD case HU178 (lanes 1) retain type 1 PrPSc after passage in Tg1014 mice (A, lane 2) and Tg22372 mice (B, lane 2). (C, D) vCJD prions in the brain of human vCJD case RU96/45 before (lanes 1) and after passage in Tg1014 (C, lanes 2–11) and Tg22372 (D, lanes 2–10) mice. The incubation period, in days, for each mouse is listed above the respective lane. vCJD prions retained type 2 PrPSc in Tg mice with longer incubation periods, but changed to type 1 PrPSc in Tg mice with shorter incubation periods. Tg mice with intermediate incubation periods (190 d in C, 279 d in D) showed a mixture of type 1 and type 2 PrPSc. Molecular weight markers represent protein standards of 20 and 30 kilodaltons.
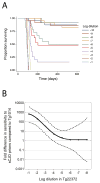
Fig. 2
(A) Kaplan-Meier survival curves for serially diluted sCJD(MM1) prions (10 tenfold dilutions of 10% brain homogenate) inoculated into Tg1014 mice. (B) Graph comparing the sensitivity of Tg1014 and Tg22372+/+ mice to log dilutions of sCJD prions. Survival data for serial dilutions of sCJD in Tg22372+/+ mice were previously reported . To compare the survival curves for each dilution in the two Tg lines, Cox regression models were used to calculate equivalent log titers. In Tg22372 mice, the 10−1 dilution resulted in a survival curve similar to that of the ~10−4 dilution inoculated into Tg1014 mice; this difference is plotted on the ordinate. An equivalent calculation was performed for each dilution in Tg22372+/+ mice. The solid line represents the interpolation of each dilution, and dashed lines represent upper and lower 95% confidence intervals.
Fig. 3
Neuropathological characterization of ill Tg1014 mice inoculated with sCJD prions (A–D) and vCJD prions (E–P). Brain sections were taken from the Tg1014 mouse inoculated with sCJD prions shown in Fig. 1A, lane 2, and Tg1014 mice inoculated with vCJD prions shown in Fig. 1C, lanes 3, 8 and 9. Tg1014 mice inoculated with sCJD prions showed moderate vacuolation (A) and punctate deposits of PrPSc (B, C), which were not amyloid (D). Tg1014 mice inoculated with vCJD, harboring type 1 PrPSc, showed moderate vacuolation similar to sCJD inoculation (E) and occasional kuru-type amyloid plaques (F–H). In mice with type 2 PrPSc, little vacuolation was observed (I) but the kuru-type amyloid plaques were large (J, K) and stained positively with Thioflavin S (L). Intermediate neuropathology was observed in Tg1014 mice with mixed type 1 and type 2 PrPSc: vacuolation was mild to moderate (M) and a few amyloid plaques were observed (N–P). Sections were stained with H&E to visualize vacuoles (left column), anti-PrP R2 antibody to visualize PrPSc deposition (middle column), and Thioflavin S to identify amyloid (right column). Bar in panel O represents 50 μm and applies panels in the three left columns; bar in panel P represents 50 μm and applies to the right column.
Fig 4
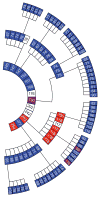
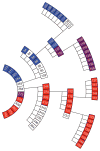
Serial passage of vCJD prions in Tg1014 mice. Each box represents a single mouse, with its incubation period in days and PrPSc strain type indicated in color: type 1 (blue), type 2 (red), types 1 and 2 (purple), not determined (white). From center, inner ring shows the first passage with human vCJD brain homogenate, middle ring shows the second passage, and outer ring shows the third passage. For each serial passage, brain homogenate from the respective ill Tg1014 mice was prepared and inoculated into other Tg1014 mice.
Fig. 5
Neuropathology resulting from serial passage of brain from vCJD-inoculated mice with either type 1 PrPSc (A–D) or type 2 PrPSc (E–H), in Tg1014 mice. For the type 1 strain, neuropathologic changes upon second (A, B) and third (C, D) passages were similar to first passage (Fig. 3E–G): moderate vacuolation (A, C) and few amyloid deposits (B, D) were observed. For type 2 PrPSc, second passage (E, F) showed little vacuolation (E) similar to first passage (Fig. 3I), but few amyloid plaques and sparse PrPSc deposits (F). Upon third passage of type 2 PrPSc (G, H), neuropathology resembled that of type 1 PrPSc with numerous vacuoles (G) and very few amyloid plaques (H). Sections stained with H & E (left column), and PrP immunohistochemistry (right column). Bar in panel H represents 50 μm and applies to all panels.
Fig. 6
Serial passage of vCJD prions in Tg22372 mice. Each box represent a single mouse, with its incubation period in days and strain type indicated in color: type 1 (blue), type 2 (red), types 1 and 2 (purple), not determined (white). From center, inner ring shows the first passage with human vCJD brain homogenate, second ring shows second passage, third ring shows third passage, outer ring shows fourth passage. For first and second serial passage, brain homogenates from the respective ill Tg22372 mice were prepared and inoculated into other hemizygous Tg22372 mice. For third and fourth passage, inoculations were performed in homozygous Tg22372+/+ mice.
Similar articles
- Abbreviated incubation times for human prions in mice expressing a chimeric mouse-human prion protein transgene.
Korth C, Kaneko K, Groth D, Heye N, Telling G, Mastrianni J, Parchi P, Gambetti P, Will R, Ironside J, Heinrich C, Tremblay P, DeArmond SJ, Prusiner SB. Korth C, et al. Proc Natl Acad Sci U S A. 2003 Apr 15;100(8):4784-9. doi: 10.1073/pnas.2627989100. Epub 2003 Apr 8. Proc Natl Acad Sci U S A. 2003. PMID: 12684540 Free PMC article. - Guinea Pig Prion Protein Supports Rapid Propagation of Bovine Spongiform Encephalopathy and Variant Creutzfeldt-Jakob Disease Prions.
Watts JC, Giles K, Saltzberg DJ, Dugger BN, Patel S, Oehler A, Bhardwaj S, Sali A, Prusiner SB. Watts JC, et al. J Virol. 2016 Oct 14;90(21):9558-9569. doi: 10.1128/JVI.01106-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27440899 Free PMC article. - Prions from Sporadic Creutzfeldt-Jakob Disease Patients Propagate as Strain Mixtures.
Cassard H, Huor A, Espinosa JC, Douet JY, Lugan S, Aron N, Vilette D, Delisle MB, Marín-Moreno A, Peran P, Beringue V, Torres JM, Ironside JW, Andreoletti O. Cassard H, et al. mBio. 2020 Jun 16;11(3):e00393-20. doi: 10.1128/mBio.00393-20. mBio. 2020. PMID: 32546613 Free PMC article. - Transgenic models of prion disease.
Scott MR, Supattapone S, Nguyen HO, DeArmond SJ, Prusiner SB. Scott MR, et al. Arch Virol Suppl. 2000;(16):113-24. doi: 10.1007/978-3-7091-6308-5_10. Arch Virol Suppl. 2000. PMID: 11214913 Review. - Prion protein transgenes and the neuropathology in prion diseases.
DeArmond SJ, Prusiner SB. DeArmond SJ, et al. Brain Pathol. 1995 Jan;5(1):77-89. doi: 10.1111/j.1750-3639.1995.tb00579.x. Brain Pathol. 1995. PMID: 7767493 Review.
Cited by
- Mouse models for studying the formation and propagation of prions.
Watts JC, Prusiner SB. Watts JC, et al. J Biol Chem. 2014 Jul 18;289(29):19841-9. doi: 10.1074/jbc.R114.550707. Epub 2014 May 23. J Biol Chem. 2014. PMID: 24860095 Free PMC article. Review. - Conserved properties of human and bovine prion strains on transmission to guinea pigs.
Safar JG, Giles K, Lessard P, Letessier F, Patel S, Serban A, Dearmond SJ, Prusiner SB. Safar JG, et al. Lab Invest. 2011 Sep;91(9):1326-36. doi: 10.1038/labinvest.2011.89. Epub 2011 Jul 4. Lab Invest. 2011. PMID: 21727894 Free PMC article. - Tau Prion Strains Dictate Patterns of Cell Pathology, Progression Rate, and Regional Vulnerability In Vivo.
Kaufman SK, Sanders DW, Thomas TL, Ruchinskas AJ, Vaquer-Alicea J, Sharma AM, Miller TM, Diamond MI. Kaufman SK, et al. Neuron. 2016 Nov 23;92(4):796-812. doi: 10.1016/j.neuron.2016.09.055. Epub 2016 Oct 27. Neuron. 2016. PMID: 27974162 Free PMC article. - Developing Therapeutics for PrP Prion Diseases.
Giles K, Olson SH, Prusiner SB. Giles K, et al. Cold Spring Harb Perspect Med. 2017 Apr 3;7(4):a023747. doi: 10.1101/cshperspect.a023747. Cold Spring Harb Perspect Med. 2017. PMID: 28096242 Free PMC article. Review. - Chimeric elk/mouse prion proteins in transgenic mice.
Tamgüney G, Giles K, Oehler A, Johnson NL, DeArmond SJ, Prusiner SB. Tamgüney G, et al. J Gen Virol. 2013 Feb;94(Pt 2):443-452. doi: 10.1099/vir.0.045989-0. Epub 2012 Oct 24. J Gen Virol. 2013. PMID: 23100369 Free PMC article.
References
- Prusiner SB. Shattuck Lecture — Neurodegenerative diseases and prions. N Engl J Med. 2001;344:1516–1526. - PubMed
- Will RG, Ironside JW, Zeidler M, et al. A new variant of Creutzfeldt-Jakob disease in the UK. Lancet. 1996;347:921–925. - PubMed
- Lasmézas CI, Deslys J-P, Demaimay R, et al. BSE transmission to macaques. Nature. 1996;381:743–744. - PubMed
- Collinge J, Sidle KCL, Meads J, et al. Molecular analysis of prion strain variation and the aetiology of "new variant" CJD. Nature. 1996;383:685–690. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 AG021601-04/AG/NIA NIH HHS/United States
- P01 AG002132/AG/NIA NIH HHS/United States
- R37 AG031220/AG/NIA NIH HHS/United States
- NS041997/NS/NINDS NIH HHS/United States
- P01 AG010770/AG/NIA NIH HHS/United States
- P01 NS041997-020003/NS/NINDS NIH HHS/United States
- R01 AI064709-03/AI/NIAID NIH HHS/United States
- P01 AG021601/AG/NIA NIH HHS/United States
- AG021601/AG/NIA NIH HHS/United States
- R01 AI064709/AI/NIAID NIH HHS/United States
- P01 AG002132-26/AG/NIA NIH HHS/United States
- AG031220/AG/NIA NIH HHS/United States
- P01 AG010770-12/AG/NIA NIH HHS/United States
- P01 NS041997/NS/NINDS NIH HHS/United States
- AI064709/AI/NIAID NIH HHS/United States
- R37 AG031220-02/AG/NIA NIH HHS/United States
- AG02132/AG/NIA NIH HHS/United States
- AG10770/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous