The role of dopamine in modulating the structure and function of striatal circuits - PubMed (original) (raw)
The role of dopamine in modulating the structure and function of striatal circuits
D James Surmeier et al. Prog Brain Res. 2010.
Abstract
Dopamine (DA) is a key regulator of action selection and associative learning. The striatum has long been thought to be a major locus of DA action in this process. Although all striatal cell types express G protein-coupled receptors for DA, the effects of DA on principal medium spiny neurons (MSNs) understandably have received the most attention. In the two principal classes of MSN, DA receptor expression diverges, with striatonigral MSNs robustly expressing D(1) receptors and striatopallidal MSNs expressing D(2) receptors. In the last couple of years, our understanding of how these receptors and the intracellular signalling cascades that they couple to modulate dendritic physiology and synaptic plasticity has rapidly expanded, fuelled in large measure by the development of new optical and genetic tools. These tools also have enabled a rapid expansion of our understanding of the striatal adaptations in models of Parkinson's disease. This chapter highlights some of the major advances in these areas.
2010 Elsevier B.V. All rights reserved.
Figures
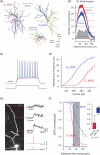
Fig. 1
D1 and D2 MSNs are differentially excitable. (a) Reconstructions of biocytin-filled D1 and D2 MSNs. Striatal neurons from P35–P45 BAC transgenic mice were biocytin filled, imaged and reconstructed in three dimensions. A GABAergic interneuron is included for comparison. (b) Analysis of anatomical differences between reconstructed D1 and D2 MSNs. A three-dimensional Sholl analysis of biocytin filled and reconstructed neurons from P35–P45 BAC transgenic mice. Data are shown as mean (±SEM) number of intersections at 1 μm eccentricities from the soma for 15 D1 and 16 D2 MSNs. D1 MSNs have a more highly branched dendritic tree, as indicated by the increased number of intersections and positive subtracted area (grey shading). (c) Membrane responses to intra-somatic current injection reveal divergence in excitability of D1 and D2 MSNs (d) The higher excitability in the D2 MSN population is illustrated in an F–I plot. (e) Maximum intensity projection image of a D2 MSN using 2PLSM (left) loaded with Alexa Fluor 568 and Fluo-4. Somatic APs were induced and corresponding spine calcium transients were measured at three distances from the soma (line scans indicated by yellow lines) and shown to the right. (f) The decrementation of somatic AP-induced dendritic calcium transients along a dendrite is compared between D1 (n = 11) and D2 (n = 6) MSNs. The data show bAP invasion into MSN dendrites degrades faster in D1 vs. D2 MSNs (Mann–Whitney rank sum test). Figure modified from Day et al. (2008) and Gertler et al. (2008).
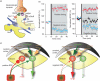
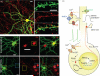
Fig. 2
STDP in D1 and D2 MSNs. (a) Model of a typical MSN dendritic spine, showing glutamatergic and dopaminergic inputs. (b) (Left) Positive spike timing (theta burst patterns of pre- and post-synaptic stimulation, pre-synaptic stimulation at –5 ms) produces LTP and negative spike timing (pre-synaptic stimulation at +10 ms) produces LTD in D2 MSNs. (Right) Positive spike timing produces LTP, whereas negative timing does not induce plastic changes in D1 MSNs. When D1 receptors are blocked by SCH23390, however, negative timing induced LTD is unmasked. (c) Model showing the behavioural consequences of differential corticostriatal STDP on D2 and D1 MSNs. Figure modified from Shen et al. (2008).
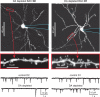
Fig. 3
Dopamine depletion causes a reduction in spine density in D2 MSNs but not D1 MSNs. Alexa 594 loaded D2 (left) and D1(right) MSNs 5 days after dopamine depletion (reserpine). High-power images of spines indicated by red boxes are shown below. After dopamine depletion spine density is significantly decreased in D2 MSNs, but appears normal in D1 MSNs. mEPSC traces taken from control and dopamine depleted MSNs (bottom) show that following dopamine depletion mEPSC frequency is decreased in D2 MSNs but unaltered in D1 MSNs, correlating with the observed change in spine density. Figure modified from Day et al. (2006).
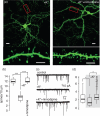
Fig. 4
L-type Ca2+ channels are necessary for spine and synapse elimination. (a) Images of D2 MSNs in corticostriatal co-cultures treated with 35 mM KCl and ionotropic receptor blockers for 24 h, in absence or presence of 10 μM nimodipine. Bar, upper panels 10 μm; lower panels, 5 μm. (b) Quantification of spine density showing that nimodipine blocked the membrane depolarization-induced spine loss (control, median = 11.9, n = 15; +K+, median = 5.6, n = 18; +K++nimodipine, median = 11.9, n = 13). (c) Examples of mEPSCs recording from the D2 MSNs treated as in (a). (d) Box plot showing membrane depolarization resulted in reduction of mEPSC frequency (control, median = 2.17, n = 19; +K+, median = 1.29, n = 14), which was blocked by nimodipine (+K++nimodipine, median = 2.92, n = 18). * p < 0.05, *** p < 0.001, Mann–Whitney rank sum test. Figure taken from Tian et al. (2010).
Fig. 5
Membrane depolarization induces MEF2-dependent Arc expression. (a) A D2 MSN in a corticostriatal co-culture treated with 35 mM KCl and ionotropic receptor blockers for 2 h and stained with anti-GFP and anti-Arc antibodies. High-magnification images (right panels) show Arc expression in dendrites. (b) Images of D2 MSNs in corticostriatal co-cultures transfected with indicated shRNAs and depolarized for 2 h. Transfected cells are shown in yellow squares, an untransfected cell is shown in a blue square. (c) Model showing the cellular signaling that mediates the spine loss in D2 MSNs. Scale bars: low-magnification images, 10 μm; high-magnification images 5 μm. Figure taken from Tian et al. (2010).
Similar articles
- Recurrent collateral connections of striatal medium spiny neurons are disrupted in models of Parkinson's disease.
Taverna S, Ilijic E, Surmeier DJ. Taverna S, et al. J Neurosci. 2008 May 21;28(21):5504-12. doi: 10.1523/JNEUROSCI.5493-07.2008. J Neurosci. 2008. PMID: 18495884 Free PMC article. - D1 and D2 dopamine-receptor modulation of striatal glutamatergic signaling in striatal medium spiny neurons.
Surmeier DJ, Ding J, Day M, Wang Z, Shen W. Surmeier DJ, et al. Trends Neurosci. 2007 May;30(5):228-35. doi: 10.1016/j.tins.2007.03.008. Epub 2007 Apr 3. Trends Neurosci. 2007. PMID: 17408758 Review. - Striatal Distribution and Cytoarchitecture of Dopamine Receptor Subtype 1 and 2: Evidence from Double-Labeling Transgenic Mice.
Ren K, Guo B, Dai C, Yao H, Sun T, Liu X, Bai Z, Wang W, Wu S. Ren K, et al. Front Neural Circuits. 2017 Aug 17;11:57. doi: 10.3389/fncir.2017.00057. eCollection 2017. Front Neural Circuits. 2017. PMID: 28860974 Free PMC article. - Differential excitability and modulation of striatal medium spiny neuron dendrites.
Day M, Wokosin D, Plotkin JL, Tian X, Surmeier DJ. Day M, et al. J Neurosci. 2008 Nov 5;28(45):11603-14. doi: 10.1523/JNEUROSCI.1840-08.2008. J Neurosci. 2008. PMID: 18987196 Free PMC article. - Differential striatal spine pathology in Parkinson's disease and cocaine addiction: a key role of dopamine?
Villalba RM, Smith Y. Villalba RM, et al. Neuroscience. 2013 Oct 22;251:2-20. doi: 10.1016/j.neuroscience.2013.07.011. Epub 2013 Jul 16. Neuroscience. 2013. PMID: 23867772 Free PMC article. Review.
Cited by
- Receptor-Independent Therapies for Forensic Detainees with Schizophrenia-Dementia Comorbidity.
Sfera A, Andronescu L, Britt WG, Himsl K, Klein C, Rahman L, Kozlakidis Z. Sfera A, et al. Int J Mol Sci. 2023 Oct 31;24(21):15797. doi: 10.3390/ijms242115797. Int J Mol Sci. 2023. PMID: 37958780 Free PMC article. - Synaptically driven state transitions in distal dendrites of striatal spiny neurons.
Plotkin JL, Day M, Surmeier DJ. Plotkin JL, et al. Nat Neurosci. 2011 Jun 12;14(7):881-8. doi: 10.1038/nn.2848. Nat Neurosci. 2011. PMID: 21666674 Free PMC article. - Dopaminergic basis for impairments in functional connectivity across subdivisions of the striatum in Parkinson's disease.
Bell PT, Gilat M, O'Callaghan C, Copland DA, Frank MJ, Lewis SJ, Shine JM. Bell PT, et al. Hum Brain Mapp. 2015 Apr;36(4):1278-91. doi: 10.1002/hbm.22701. Epub 2014 Nov 25. Hum Brain Mapp. 2015. PMID: 25425542 Free PMC article. - Computational Characteristics of the Striatal Dopamine System Described by Reinforcement Learning With Fast Generalization.
Fujita Y, Yagishita S, Kasai H, Ishii S. Fujita Y, et al. Front Comput Neurosci. 2020 Jul 22;14:66. doi: 10.3389/fncom.2020.00066. eCollection 2020. Front Comput Neurosci. 2020. PMID: 32774245 Free PMC article. - Mechanisms of hierarchical reinforcement learning in corticostriatal circuits 1: computational analysis.
Frank MJ, Badre D. Frank MJ, et al. Cereb Cortex. 2012 Mar;22(3):509-26. doi: 10.1093/cercor/bhr114. Epub 2011 Jun 21. Cereb Cortex. 2012. PMID: 21693490 Free PMC article.
References
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends in Neurosciences. 1989;12:366–375. - PubMed
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: Neural substrates of parallel processing. Trends in Neurosciences. 1990;13:266–271. - PubMed
- Ariano MA. Distribution of components of the guanosine 3’,5’-phosphate system in rat caudate-putamen. Neuroscience. 1983;10:707–723. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous