Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input - PubMed (original) (raw)
Comparative Study
Rapid desynchronization of an electrically coupled interneuron network with sparse excitatory synaptic input
Koen Vervaeke et al. Neuron. 2010.
Abstract
Electrical synapses between interneurons contribute to synchronized firing and network oscillations in the brain. However, little is known about how such networks respond to excitatory synaptic input. To investigate this, we studied electrically coupled Golgi cells (GoC) in the cerebellar input layer. We show with immunohistochemistry, electron microscopy, and electrophysiology that Connexin-36 is necessary for functional gap junctions (GJs) between GoC dendrites. In the absence of coincident synaptic input, GoCs synchronize their firing. In contrast, sparse, coincident mossy fiber input triggered a mixture of excitation and inhibition of GoC firing and spike desynchronization. Inhibition is caused by propagation of the spike afterhyperpolarization through GJs. This triggers network desynchronization because heterogeneous coupling to surrounding cells causes spike-phase dispersion. Detailed network models predict that desynchronization is robust, local, and dependent on synaptic input properties. Our results show that GJ coupling can be inhibitory and either promote network synchronization or trigger rapid network desynchronization depending on the synaptic input.
(c) 2010 Elsevier Inc. All rights reserved.
Figures
Figure 1
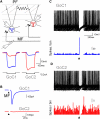
Mossy Fiber Stimulation Can Excite or Inhibit Golgi Cell Firing (A) Schematic diagram of paired Golgi cell (GoC) recording configuration with mossy fiber (MF) stimulation (PF; parallel fiber input). Voltage responses recorded in a cell pair in response to injected current pulses (−0.2 nA) in one of the cells. The coupling coefficient (CC) was 19.4%. (B) MF stimulation produced an EPSC only in GoC1 (Vhold = −70 mV) in the presence of 10 μM gabazine and 0.5 μM strychnine to block inhibition. (C) Superimposed voltage recordings showing firing of GoC1 during single shock MF stimulation (same stimuli as in B), which reliably evoked a spike followed by a pause of firing. Bottom panel shows spike histograms (10 ms bins). (D) Superimposed voltage recordings showing firing of GoC2 during the same trials as (C), together with spike histograms below. GoC2 responded to MF input into GoC1 with an inhibitory pause. See also Figure S1.
Figure 2
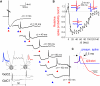
Time Course of Electrical Excitation and Inhibition in Golgi Cells (A) (Inset) Schematic diagram showing recording from two Golgi cells (GoCs) and the timing protocol of injected EPSC-like current waveforms (iEPSC). The iEPSC in GoC1 was scaled to reliably evoke a spike, while the iEPSC in GoC2 was scaled to evoke spikes with a probability of ∼0.7. The coupling coefficient (CC) was 16.3%. A small steady negative current (10–20 pA) was injected to prevent spontaneous firing. From top to bottom: individual voltage responses of GoC2 for different intervals (Δt) between iEPSC injection into the two cells. At Δt = 0 and 150 ms the iEPSC caused a spike (truncated for clarity), but at intermediate times the GJ potential inhibited spike generation in GoC2. (B) Relative spike probability of GoC2 as a function of Δt (for Δt > 100 ms, n = 4 cell pairs; for Δt < 100, n = 9 cell pairs). Insets show examples of the membrane voltage traces of GoC1 (blue) and GoC2 (red) for Δt of 0, 40, and 130 ms. Note that GoC2 fails to spike when Δt = 40 ms. Data represent mean ± SE. (C) Example of a spike in GoC1 and the spikelet in GoC2, normalized to the peak. See also Figure S2.
Figure 3
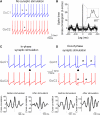
Sparse Synchronous Synaptic Input Can Cause Spike Desynchronization (A) Voltage traces from a Golgi cell (GoC) pair (coupling coefficient [CC]: 23.1%) in the absence of synaptic stimulation. Spikes were synchronized, but they occasionally skipped a cycle (asterisks) producing transient antiphase firing. (B) Cross-correlogram of spike times for the same cells as in (A). Horizontal lines indicate confidence intervals. Inset shows the central peak at an expanded timescale. (C) Voltage traces from another GoC pair (CC: 19.4%). Stimulation of mossy fiber (MF) input into GoC1 (arrowhead) in phase with a spike did not perturb spike synchronization between GoC1 and GoC2. (Bottom panels) Cross-correlation of the spike time distribution of both cells across multiple repeated trials, in a 300 ms time window before stimulation (left), and in a 400 ms time window after the synaptic stimulation (right). Only sweeps where both GoCs showed spike synchrony before stimulation were included. Inhibitory synaptic transmission was blocked with 10 μM gabazine and 0.5 μM strychnine. (D) Out-of-phase stimulation of the same MF input into GoC1 caused antiphase firing and short-term spike desynchronization, as indicated by the trough in the cross-correlation plot below. Note that after stimulation both cells mutually inhibit each other by the propagation of their spike AHP (asterisks) as can be seen occurring also spontaneously in (A).
Figure 4
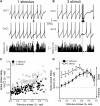
Golgi Cell Inhibitory Responses to Bursts of Synaptic Input (A) Voltage traces from a Golgi cell (GoC) pair (coupling coefficient [CC]: 23.9%) during mossy fiber (MF) input into one of the cells. Inhibitory synaptic transmission was blocked by 10 μM gabazine and 0.5 μM strychnine. A single MF stimulus reliably evoked a spike in GoC1, while causing a pause in the firing of GoC2. (Bottom panel) Spike histogram of GoC2 (10 ms bins). (B) In the same cell pair, three stimuli at 33 Hz evoked multiple spikes in GoC1 causing longer pauses in firing of GoC2. (C) Individual phase response curves for cell pair in (A) and (B), where the average interspike interval corresponds to a full cycle (2π radians). MF stimulation delayed the phase of spikes in GoC2 as a function of the stimulation phase for one and three MF stimuli. (D) Average phase response curves for one and three MF stimuli (n = 5 cell pairs; p < 0.05 when stimulus phase < 0.65). Data represent mean ± SE. See also Figure S3.
Figure 5
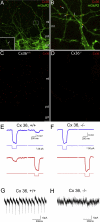
Connexin-36 Mediates Electrical Coupling between Golgi Cell Dendrites (A and B) Double immunofluorescent reactions for Cx36 (red) and the selective Golgi Cell (GoC) marker mGluR2 (green) in mouse cerebellar cortex. Some Cx36-immunoreactive puncta were detected at the intersections of two mGluR2-immunopositive dendrites (arrows). The majority of Cx36-immunopositive puncta were not associated with mGluR2-immunopositive dendrites (double arrowheads). Inset in (A) is a high-magnification view of the boxed area. Inset in (A) is a single confocal section. Rest: maximum intensity Z projection of (A, 11; B, 8) confocal sections (at A, 2 μm; B, 0.5 μm separation). (C and D) Immunofluorescent labeling of Cx36 in the cerebellar cortex of a wild-type mouse (Cx36+/+, C) and a Cx36 knockout littermate (_Cx36_−/−, D). (E and F) Voltage responses of a GoC pair from a Cx36+/+ (E) and a _Cx36_−/− mouse (F) in response to injected current pulses in one of the cells. A small hyperpolarizing current (5–60 pA) was applied to stop the cells from spontaneous firing. (G and H) Voltage-clamp recordings with a Cs-gluconate-based internal solution containing QX314 and tetraethylammonium (TEA) showing rapid inward spikelets followed by slower outward currents from single GoCs. Cells were held depolarized at −30 to +30 mV. Spikelets were observed in Cx36+/+ but not in _Cx36_−/− mice. Scale bars: (A, C, and D) 10 μm, (A, inset, and B) 2 μm, (C) and (D) are at the same magnification. pcl, Purkinje cell layer; gcl, granule cell layer; ml, molecular layer. See also Figure S4.
Figure 6
Electron Microscopic Localization of Gap Junctions between Two Golgi Cells and Compartmental Modeling of Their Electrophysiological Behavior (A) (Left) Light microscopic reconstruction of two electrically coupled Golgi cells (GoCs) previously whole-cell recorded and filled with biocytin (GoC1: soma and dendrites, blue; axon, green; GoC2: soma and dendrites, red; axon, black). (Right) High-magnification view of the gap junction locations (GJs, arrows). Other dendrites are not shown for clarity. (B and C) Electron micrographs of two GJs (gj03 and gj06) formed by dendrites d1 and d2. (D and E) High-magnification images of the GJs (arrowheads) shown in (B) and (C). (F–K) Simulations with the reconstructed morphologies of the coupled GoC pair. The nine GJs were inserted at positions determined by electron microscopy. (F) Comparison of the model and the experimental responses (from the same GoC pair) to 200 ms current pulses (−200 pA). Throughout (F) and (G), thin lines are five experimental responses; thick lines are the model predictions. During the experiments and in the model, steady negative current was applied to stop the cells from rhythmic firing (typically −5 to −100 pA, baseline Vm in model and experiment: ∼−55 mV). The conductances of the GJs were adjusted to match the voltage attenuation from the blue to the red GoC (left panels; each GJ = 130 pS). Note that this did not match perfectly the attenuation from the red to the blue GoC (right panels). Experimental CC from blue to red = 28.2%, CC from red to blue = 15.4%. (G) (Left) Experimental and model spikes and AHPs (during spontaneous firing at ∼8 Hz). Inset shows spikes on an expanded timescale. (Right) Experimental and model GJ potentials. (H) Model voltage responses showing spike synchrony in the absence of synaptic input and occasional missed spikes (asterisks). (I) Model spike train responses (black, 150 superimposed traces) and spike histograms (10 ms bins) of both GoCs. GoCs were spontaneously spiking at ∼8 Hz. Simultaneous activation of 20 mossy fiber (MF) synapses on the blue GoC (arrowhead) reliably evoked a spike followed by a pause, while only causing a pause in the firing of the red GoC. (J) Simulations showing that out-of-phase MF input (arrow) to the blue GoC caused transient antiphase firing in the cell pair. (K) Model phase response curves of red GoC when the blue GoC is stimulated with MF input (as in I and J). In this simulation the conductance of the GJs was adjusted to give a CC of 9% and 23% in order to compare with experimental data in Figure S3C; note that due to the smaller bin-width of the model data, the phase advance for spikes later in the cycle is more pronounced than for the experimental data. ml, molecular layer; pcl, Purkinje cell layer; gcl, granule cell layer. Scale bars: (A) 50 μm, (A, inset) 10 μm, (B and C) 250 nm, (D and E) 50 nm. See also Table S1 and Figure S5.
Figure 7
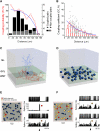
Sparse Excitatory Inputs Evoke Gap Junction-Mediated Surround Inhibition in a GoC Network Model (A) Experimentally measured coupling probabilities (Pc) between pairs of Golgi cells (GoCs) as a function of distance between their somata (136 pairs). The Pc is fitted with a Boltzmann function (blue, y = −1745 + 1836/{1 + exp[(distance − 267)/39]}). Histogram shows the number of coupled and noncoupled GoC pairs (20 μm bins). (B) Experimentally measured coupling coefficient (CC, circles) as a function of distance between somata (174 pairs). Histogram shows the binned CC (10 μm bins). CC data are fitted with a single exponential decay function [blue, y = −2.3 + 29.7 ∗ exp(–distance/70.4)]. Column bars represent mean ± SE. (C) The 3D volume of the network model comprising white matter tract (WMT) granule cell layer (GCL) and molecular layer (ML) together with reconstructed GoC used in the network model (soma and dendrites, blue; axon, red). (D) Forty-five randomly distributed GoCs in a modeled slab of GCL of 350 × 350 × 80 μm; dendrites and axons are omitted for clarity. (E) (Left) Top view of the 3D model. Temporally precise synaptic input to a random GoC (red, Experimental Procedures) evoked an excitation followed by a pause while three neighboring GoCs (yellow) that did not receive synaptic input showed pause-only responses in their spontaneous spiking. (Right) Top two panels (red cell): superimposed spike trains (100 traces) and spike histogram (10 ms bins). Bottom two panels show a typical yellow cell pause. (F) (Left) The same synaptic input as in (E) to ten random GoCs (∼22% of the network) triggered an excitation followed by a pause in the red GoCs while 27 GoCs responded only with a pause in spontaneous spiking (yellow). (Right) Top two panels: the majority of inhibited GoCs responded with a pure pause (yellow, “x”); bottom two panels: a minority of inhibited GoCs showed a small peak before the pause in the spike histogram (yellow, “+”). See also Figure S6.
Figure 8
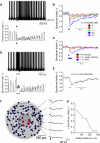
Simulated Sparse Excitatory Inputs Evoke a Transient Local Network Desynchronization (A) (Top) Superimposed spike trains of all 45 Golgi cells (GoCs) in the network. During the baseline period, the GoCs show loose spike synchrony at a population frequency of ∼8 Hz. When ten random GoCs (∼22% of the network) were excited by suprathreshold synaptic input (arrowhead, same input as in Figures 7E and 7F; Experimental Procedures) the GoCs temporarily desynchronized. (Bottom) The temporal evolution of the spike synchrony determined by the synchrony index, SI(t). SI(t) is the total number of spikes within t and t + 20 ms normalized by the number of cells. Thin line connects the rhythmic ∼8 Hz peaks of the SI(t). (B) The dependence of spike desynchronization on the percentage of excited GoCs. After a baseline period, a different number of GoCs received synaptic excitation (arrow). Connected squares show the temporal evolution of the spike synchrony (curves are obtained as illustrated by the thin line in A). Each point (squares) show mean ± SE for ten different randomly connected networks. (C) The dependence of spike desynchronization on the level and duration of the stimulus rate (n = 10). Note that for plots in (B) and (C) the stimulus-evoked spikes were omitted. (D) Example of spike desynchronization during a 250 ms train of asynchronous synaptic input (Experimental Procedures). (E) Mean time course of spike desynchronization (ten networks). (F) (Left) Top view of a cylindrical network (diameter 600 μm, 104 GoCs). Central area is 100 μm in radius and concentric rings are 40 μm wide. Red GoCs received a suprathreshold synaptic input. Right panels show the temporal evolution of the synchrony index following the excitation of the red GoCs (arrow). Traces are averaged over nine network instantiations. (G) Summary of the change in spike synchrony index from the data in (F). See also Figure S7.
Figure 9
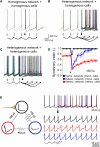
Determinants of Network Desynchronization (A–C) Superimposed voltage traces from 45 Golgi cells (GoCs) in three different network configurations. Ten randomly selected Golgi cells (22%) were excited by supra threshold synaptic input (arrowhead; same as in Figure 8A). (A) Model with spatially homogeneous all-to-all connectivity and GJs of equal conductance between the same location on the ascending dendrites of GoCs with homogeneous cellular properties (each cell has the same input resistance and spontaneous firing rate). (B) Model with spatially heterogeneous connection probability and coupling coefficients as measured (see Figures 7A and 7B), but with homogeneous GoCs. (C) Model with spatially heterogeneous network properties and heterogeneous GoCs (cells have different input resistance and spontaneous firing rates as used in Figures 7 and 8) as observed for real GoCs. (D) Time course of network synchrony after a synaptic input (arrowhead) for the models in (A) (black symbols), (B) (blue symbols), and (C) (red symbols). Data represent mean ± SE. Note that for plots in (D) the stimulus-evoked spikes were omitted. (E) Cartoon of a minimal GoC network showing desynchronization: three identical GoCs coupled together at the soma by electrical synapses with heterogeneous conductances. Only the black cell received synaptic input. (Right) Voltage traces from the black, red, and blue cell. Timing of suprathreshold synaptic input into the black cell indicated by the arrowhead. Spikes truncated in lower three traces for clarity.
Comment in
- Enhanced functions of electrical junctions.
Connors BW, Zolnik TA, Lee SC. Connors BW, et al. Neuron. 2010 Aug 12;67(3):354-6. doi: 10.1016/j.neuron.2010.07.024. Neuron. 2010. PMID: 20696372 Free PMC article.
Similar articles
- Active Dendrites and Differential Distribution of Calcium Channels Enable Functional Compartmentalization of Golgi Cells.
Rudolph S, Hull C, Regehr WG. Rudolph S, et al. J Neurosci. 2015 Nov 25;35(47):15492-504. doi: 10.1523/JNEUROSCI.3132-15.2015. J Neurosci. 2015. PMID: 26609148 Free PMC article. - Regulating synchronous oscillations of cerebellar granule cells by different types of inhibition.
Tang Y, An L, Wang Q, Liu JK. Tang Y, et al. PLoS Comput Biol. 2021 Jun 28;17(6):e1009163. doi: 10.1371/journal.pcbi.1009163. eCollection 2021 Jun. PLoS Comput Biol. 2021. PMID: 34181653 Free PMC article. - Gap junctions compensate for sublinear dendritic integration in an inhibitory network.
Vervaeke K, Lorincz A, Nusser Z, Silver RA. Vervaeke K, et al. Science. 2012 Mar 30;335(6076):1624-8. doi: 10.1126/science.1215101. Epub 2012 Mar 8. Science. 2012. PMID: 22403180 Free PMC article. - Unraveling the cerebellar cortex: cytology and cellular physiology of large-sized interneurons in the granular layer.
Geurts FJ, De Schutter E, Dieudonné S. Geurts FJ, et al. Cerebellum. 2003;2(4):290-9. doi: 10.1080/14734220310011948. Cerebellum. 2003. PMID: 14964688 Review. - Interneuron diversity series: inhibitory interneurons and network oscillations in vitro.
Whittington MA, Traub RD. Whittington MA, et al. Trends Neurosci. 2003 Dec;26(12):676-82. doi: 10.1016/j.tins.2003.09.016. Trends Neurosci. 2003. PMID: 14624852 Review.
Cited by
- Computational models of neurotransmission at cerebellar synapses unveil the impact on network computation.
Masoli S, Rizza MF, Tognolina M, Prestori F, D'Angelo E. Masoli S, et al. Front Comput Neurosci. 2022 Oct 28;16:1006989. doi: 10.3389/fncom.2022.1006989. eCollection 2022. Front Comput Neurosci. 2022. PMID: 36387305 Free PMC article. Review. - A novel inhibitory nucleo-cortical circuit controls cerebellar Golgi cell activity.
Ankri L, Husson Z, Pietrajtis K, Proville R, Léna C, Yarom Y, Dieudonné S, Uusisaari MY. Ankri L, et al. Elife. 2015 May 12;4:e06262. doi: 10.7554/eLife.06262. Elife. 2015. PMID: 25965178 Free PMC article. - ModelView for ModelDB: Online Presentation of Model Structure.
McDougal RA, Morse TM, Hines ML, Shepherd GM. McDougal RA, et al. Neuroinformatics. 2015 Oct;13(4):459-70. doi: 10.1007/s12021-015-9269-2. Neuroinformatics. 2015. PMID: 25896640 Free PMC article. - Open Source Brain: A Collaborative Resource for Visualizing, Analyzing, Simulating, and Developing Standardized Models of Neurons and Circuits.
Gleeson P, Cantarelli M, Marin B, Quintana A, Earnshaw M, Sadeh S, Piasini E, Birgiolas J, Cannon RC, Cayco-Gajic NA, Crook S, Davison AP, Dura-Bernal S, Ecker A, Hines ML, Idili G, Lanore F, Larson SD, Lytton WW, Majumdar A, McDougal RA, Sivagnanam S, Solinas S, Stanislovas R, van Albada SJ, van Geit W, Silver RA. Gleeson P, et al. Neuron. 2019 Aug 7;103(3):395-411.e5. doi: 10.1016/j.neuron.2019.05.019. Epub 2019 Jun 11. Neuron. 2019. PMID: 31201122 Free PMC article. - The contribution of extrasynaptic signaling to cerebellar information processing.
Coddington LT, Nietz AK, Wadiche JI. Coddington LT, et al. Cerebellum. 2014 Aug;13(4):513-20. doi: 10.1007/s12311-014-0554-7. Cerebellum. 2014. PMID: 24590660 Free PMC article. Review.
References
- Beierlein M., Gibson J.R., Connors B.W. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat. Neurosci. 2000;3:904–910. - PubMed
Publication types
MeSH terms
Grants and funding
- 086699/WT_/Wellcome Trust/United Kingdom
- G0400598/MRC_/Medical Research Council/United Kingdom
- F005490/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F005490/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous