The blind leading the obese: the molecular pathophysiology of a human obesity syndrome - PubMed (original) (raw)
Review
. 2010:121:172-81; discussion 181-2.
Affiliations
- PMID: 20697559
- PMCID: PMC2917141
Review
The blind leading the obese: the molecular pathophysiology of a human obesity syndrome
Val C Sheffield. Trans Am Clin Climatol Assoc. 2010.
Abstract
Bardet-Biedl syndrome (BBS) is a genetically heterogeneous disorder affecting multiple organ systems and resulting in blindness, obesity, cognitive impairment, and congenital defects. Interest in the etiology of this disorder stems, in part, from the fact that patients with BBS develop common clinical problems, including obesity, diabetes and hypertension. Twelve genes independently causing BBS have been identified. The heterogeneity is explained by the existence of two BBS complexes, the BBSome consisting of seven known BBS proteins, and the BBS chaperone complex consisting of three known BBS proteins. The formation of the BBSome requires the function of the BBS chaperone complex. Both mouse and zebrafish data support a role for BBS genes in cilia function, and in intracellular and intraflagellar trafficking. From the work described here, a common primary function of BBS proteins has emerged, specifically the mediation and regulation of microtubule-based intracellular transport.
Conflict of interest statement
Potential Conflicts of Interest: None disclosed.
Figures
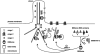
Fig. 1
Schematic drawing of the BBSome complex showing protein-protein interactions. BBS1, BBS2, BBS7 and BBS9 contain Beta-propeller repeats. BBS4 and BBS8 contain TPR repeats.
Fig. 2
Histological samples from control (left) and BBS knockout (right) mouse testes. Spermatozoa from BBS mice (Bbs4 knockout mouse shown) lack flagella.
Fig. 3
Histological slides of retina from eight month old wild-type (left) and Bbs4 knockout (right) mice. Note that Bbs4-/- mice lack the photoreceptor layer below the retinal pigmented epithelium (RPE). The photoreceptor layer has completely degenerated in these mice.
Fig. 4
BBS knockout mouse showing the obesity phenotype.
Fig. 5
BBS working model. Seven BBS proteins form a stable complex (BBSome) which localizes to centriolar satellites and to the membrane of cilia. BBSome components play a role in regulation of protein and vesicle trafficking to cilia, and perhaps to the plasma membrane. Chaperone-like BBS proteins (BBS6, BBS10 and BBS12) form a second BBS complex that is required for BBSome assembly.
Similar articles
- BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking.
Zhang Q, Nishimura D, Vogel T, Shao J, Swiderski R, Yin T, Searby C, Carter CS, Kim G, Bugge K, Stone EM, Sheffield VC. Zhang Q, et al. J Cell Sci. 2013 Jun 1;126(Pt 11):2372-80. doi: 10.1242/jcs.111740. Epub 2013 Apr 9. J Cell Sci. 2013. PMID: 23572516 Free PMC article. - Intrinsic protein-protein interaction-mediated and chaperonin-assisted sequential assembly of stable bardet-biedl syndrome protein complex, the BBSome.
Zhang Q, Yu D, Seo S, Stone EM, Sheffield VC. Zhang Q, et al. J Biol Chem. 2012 Jun 8;287(24):20625-35. doi: 10.1074/jbc.M112.341487. Epub 2012 Apr 12. J Biol Chem. 2012. PMID: 22500027 Free PMC article. - Bardet-Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes.
Zhang Q, Nishimura D, Seo S, Vogel T, Morgan DA, Searby C, Bugge K, Stone EM, Rahmouni K, Sheffield VC. Zhang Q, et al. Proc Natl Acad Sci U S A. 2011 Dec 20;108(51):20678-83. doi: 10.1073/pnas.1113220108. Epub 2011 Dec 2. Proc Natl Acad Sci U S A. 2011. PMID: 22139371 Free PMC article. - Bardet-Biedl syndrome: an emerging pathomechanism of intracellular transport.
Blacque OE, Leroux MR. Blacque OE, et al. Cell Mol Life Sci. 2006 Sep;63(18):2145-61. doi: 10.1007/s00018-006-6180-x. Cell Mol Life Sci. 2006. PMID: 16909204 Free PMC article. Review. - Molecular basis of the obesity associated with Bardet-Biedl syndrome.
Guo DF, Rahmouni K. Guo DF, et al. Trends Endocrinol Metab. 2011 Jul;22(7):286-93. doi: 10.1016/j.tem.2011.02.009. Epub 2011 Apr 21. Trends Endocrinol Metab. 2011. PMID: 21514177 Free PMC article. Review.
Cited by
- Cycling of the signaling protein phospholipase D through cilia requires the BBSome only for the export phase.
Lechtreck KF, Brown JM, Sampaio JL, Craft JM, Shevchenko A, Evans JE, Witman GB. Lechtreck KF, et al. J Cell Biol. 2013 Apr 15;201(2):249-61. doi: 10.1083/jcb.201207139. J Cell Biol. 2013. PMID: 23589493 Free PMC article. - CP110 and its network of partners coordinately regulate cilia assembly.
Tsang WY, Dynlacht BD. Tsang WY, et al. Cilia. 2013 Jul 26;2(1):9. doi: 10.1186/2046-2530-2-9. Cilia. 2013. PMID: 24053599 Free PMC article. - IFT-Cargo Interactions and Protein Transport in Cilia.
Lechtreck KF. Lechtreck KF. Trends Biochem Sci. 2015 Dec;40(12):765-778. doi: 10.1016/j.tibs.2015.09.003. Epub 2015 Oct 21. Trends Biochem Sci. 2015. PMID: 26498262 Free PMC article. Review. - A novel H395R mutation in MKKS/BBS6 causes retinitis pigmentosa and polydactyly without other findings of Bardet-Biedl or McKusick-Kaufman syndrome.
Hulleman JD, Nguyen A, Ramprasad VL, Murugan S, Gupta R, Mahindrakar A, Angara R, Sankurathri C, Mootha VV. Hulleman JD, et al. Mol Vis. 2016 Jan 24;22:73-81. eCollection 2016. Mol Vis. 2016. PMID: 26900326 Free PMC article. - TUDCA slows retinal degeneration in two different mouse models of retinitis pigmentosa and prevents obesity in Bardet-Biedl syndrome type 1 mice.
Drack AV, Dumitrescu AV, Bhattarai S, Gratie D, Stone EM, Mullins R, Sheffield VC. Drack AV, et al. Invest Ophthalmol Vis Sci. 2012 Jan 5;53(1):100-6. doi: 10.1167/iovs.11-8544. Invest Ophthalmol Vis Sci. 2012. PMID: 22110077 Free PMC article.
References
- Laurence JZ, Moon RC. Four cases of retinitis pigmentosa occurring in the same family, and accompanied by general imperfections of development. Ophthal Rev. 1866;2:32–41. - PubMed
- Bardet G. Thesis. Paris: University of Paris; 1920. Sur un syndrome d'obesite' congenitale avec polydactylie et retinite pigmentaire (contribution a l'etude des formes cliniques de l'obesite hypophysaire)
- Biedl A. Ein Geschwisterpaar mir adipose-genitaler Dystrophie. Dtsch Med Wochenschr. 1922;48:1630.
- Solis-Cohens S, Weiss E. The Laurence Biedl syndrome: a report of four cases in one family. Am J Med Sci. 1925;169:489.
- Hrynchak PK. Bardet-Biedl syndrome. Optom Vis Sci. 2000;77:236–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical