Monoamine oxidases regulate telencephalic neural progenitors in late embryonic and early postnatal development - PubMed (original) (raw)
Monoamine oxidases regulate telencephalic neural progenitors in late embryonic and early postnatal development
Aiwu Cheng et al. J Neurosci. 2010.
Abstract
Monoamine neurotransmitters play major roles in regulating a range of brain functions in adults and increasing evidence suggests roles for monoamines in brain development. Here we show that mice lacking the monoamine metabolic enzymes MAO A and MAO B (MAO AB-deficient mice) exhibit diminished proliferation of neural stem cells (NSC) in the developing telencephalon beginning in late gestation [embryonic day (E) 17.5], a deficit that persists in neonatal and adult mice. These mice showed significantly increased monoamine levels and anxiety-like behaviors as adults. Assessments of markers of intermediate progenitor cells (IPC) and mitosis showed that NSC in the subventricular zone (SVZ), but not in the ventricular zone, are reduced in MAO AB-deficient mice. A developmental time course of monoamines in frontal cortical tissues revealed increased serotonin levels as early as E14.5, and a further large increase was found between E17.5 and postnatal day 2. Administration of an inhibitor of serotonin synthesis (parachlorophenylalanine) between E14.5 and E19.5 restored the IPC numbers and SVZ thickness, suggesting the role of serotonin in the suppression of IPC proliferation. Studies of neurosphere cultures prepared from the telencephalon at different embryonic and postnatal ages showed that serotonin stimulates proliferation in wild-type, but not in MAO AB-deficient, NSC. Together, these results suggest that a MAO-dependent long-lasting alteration in the proliferation capacity of NSC occurs late in embryonic development and is mediated by serotonin. Our findings reveal novel roles for MAOs and serotonin in the regulation of IPC proliferation in the developing brain.
Figures
Figure 1.
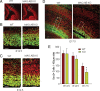
Numbers of neural progenitor cells in the germinal zone of the telencephalon are significantly reduced late in embryonic development in MAO AB-deficient mice. A–D, Coronal brain sections from E12.5 (A, B), E14.5 (C), and E17.5 (D) WT and MAO AB KO littermate mice taken from matched sections at the same level of frontal cortex were immunostained with a Sox2 antibody (green) and counterstained with PI (red). Representative higher-magnification confocal images of a slab of the middle telencephalon wall as indicated in A are shown in B–D. Scale bar, 50 μm. E, The total number of Sox2+ cells in 100 μm slab were quantified and plotted. *p < 0.5 (n = 4 mice).
Figure 2.
MAO AB-deficient mice exhibit reduced proliferation of neural progenitor cells in the subventricular zone beginning late in embryonic development. Pregnant dams at E12.5, E14.5, and E17.5 were pulse (1 h) labeled with BrdU and the brain sections were immunostained with BrdU (green) and counterstained with PI (red). A, The distribution of the S-phase nuclei (BrdU+ cells) was analyzed by dividing the cortical VZ/SZV into 10 μm bins across the cerebral wall from the ventricle surface. Each bin was 10 × 100 μm in size. BrdU+ and PI+ cells in each bin were counted and the LI (BrdU+/PI+) in each bin was calculated. B–D, Representative confocal images of a slab of the middle telencephalon wall of WT and MAO AB KO mice at E12.5 (B), E14.5 (C), and E17.5 (D). Scale bar, 50 μm. E–G, The LI was plotted for each of the bins within the analysis areas at E12.5 (E), E14.5 (F), and E17.5 (B). At E17.5, BrdU+ cells were significantly reduced in the area corresponding to subventricular zone area, but not in the ventricular zone. *p < 0.05 (n = 4 mice).
Figure 3.
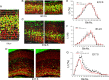
Mice lacking MAO A and MAO B exhibit a sustained reduction in neural progenitor cell proliferation in the subventricular zone during early postnatal and adult life. A–C, Neonatal mice (P2) were administrated BrdU and killed 1 h later. Brain sections were prepared and immunostained with a BrdU antibody (green) and counterstained with PI (red). Representative confocal images of BrdU staining in the SVZ of the frontal cortex in WT and MAO AB KO neonatal mice are presented (A) and higher magnification of the dorsal telencephalon SVZ immunostained with BrdU (A, dotted square 1) are shown (B). Scale bar, 50 μm. C, BrdU+ cells in a 200 μm slab of dorsal telencephalon SVZ were quantified and plotted. **p < 0.01 (n = 4 mice). D, Higher magnifications of the LGE SVZ immunostained with BrdU antibody (dotted square 2). E, BrdU+ cells in a 200 μm slab of LGE SVZ from WT and MAO AB KO mice are shown. Scale bar, 50 μm. F, Results of counts of BrdU-labeled cells in the SVZ of P2 WT and MAO AB KO mice. **p < 0.01 (n = 4 mice). G, Adult mice were administered three pulses of BrdU at 2 h intervals. Every sixth section in the forebrain was processed for BrdU immunohistochemistry. All BrdU-positive cells in the SVZ surrounding the lateral ventricle in each section were counted for each brain. The values represent the total number of BrdU-positive cells at designated level for each brain. **p < 0.01 (n = 4 mice).
Figure 4.
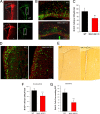
IPC, but not RGCs in VZ, are specifically affected in MAO AB KO mice. A, Representative confocal images of TBR2 immunostaining (green) in brain sections from E12.5 (a1), E14.5 (a2), and E17.5 (a3) WT mice; the sections are counterstained with PI (red). TBR2 is a marker for IPC in the SVZ of the dorsal telencephalon. B, Representative confocal images of TBR2 (green) and PCNA (red) double immunostaining in WT E17.5 frontal cortex (b1) and higher magnification (b2, PCNA staining; b3, merged image of PCNA and TBR2 staining) showed that nearly all TBR2+ cells are also PCNA+. Arrows, Representative cells. C, The percentage of TBR2+/Sox2+ cells in a 100 μm slab was plotted for different development stages. VZ cells gradually decrease in numbers, whereas the SVZ expands as development proceeds. D, E, Coronal sections of E17.5 and P2 frontal cortex of WT and MAO AB KO mice immunostained with anti-TBR2 (D, green) or anti-PH3 (E, green; a marker for cell entry into mitosis). Sections were counterstained with PI (D, E, red). Note that PH3 immunostaining clearly demonstrates two types of dividing cells: surface dividing and non-surface dividing. F, Numbers of non-surface dividing TBR2+ cells in the dorsal telencephalon at the indicated embryonic developmental stages. G, Numbers of surface dividing and non-surface dividing PH3+ cells at the indicated embryonic and postnatal developmental stages. Note that numbers of non-surface dividing TBR2+ cells and PH3+ cells are significantly reduced in E17.5 and P2 MAO ABKO mice compared with WT mice. *p < 0.05 (n = 4 mice).
Figure 5.
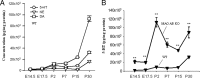
Cortical serotonin levels increase markedly during late embryonic and early postnatal brain development. A, Developmental change (E14.5–P30) in levels of 5-HT, DA, and NE in the cortex of WT mice. B, There is a dramatic increase in levels of 5-HT in the cerebral cortex of MAO AB KO mice compared with WT mice. Values are expressed as pg/mg protein and represent the mean ± SEM. **p < 0.001 (n = 3–5 mice).
Figure 6.
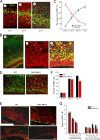
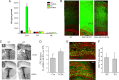
Evidence that MAO activity exerts a long-lasting effect on the proliferative capacity of NSC in the developing telencephalon. A, Images of neurospheres in cultures prepared from the VZ/SVZ of the dorsal telencephalon of E14.5, E17.5, and P2 MAO AB KO and WT littermate mice. Scale bar, 1.0 mm. B, C, Sizes of first generation (B) and second generation (C) neurospheres established from telencephalic tissues of WT and MAO AB KO mice of the indicated ages. Values are the mean and SEM (n = 3–4 mice). *p < 0.05, **p < 0.01. D, Levels of MAO A and MAO B activities in neurospheres cultured from WT and MAO AB KO mice of the indicated ages (E12.5, E15.5, and P2). Note that cultured NS exhibit MAO A, but not MAO B activity, and MAO A activity significantly increases as development proceeds. E, Levels of MAO A activity in E17.5 neurospheres measured 24 h after treatment with the indicated concentrations of clorgyline (an inhibitor of MAO A). F, G, Clorgyline administration did not significantly alter the diameter (F) or number (G) of neurospheres in cultures established from P2 cortical tissue. H, Low concentrations of serotonin (10 and 100 ng/ml) stimulate neurosphere formation in P2 cultures from WT mice, but not in cultures from MAO AB KO mice. Values are the mean and SEM (n = 3–4 mice). **p < 0.01 compared with the value for cultures not exposed to serotonin.
Figure 7.
Blocking the serotonin surge in the telencephalon of MAO AB KO mice during late embryonic and early postnatal development restores NSC proliferation and cortical thickness to normal levels. A, Serotonin levels in the cortex of P1 WT mice that had developed in pregnant dams treated with vehicle (−) or the serotonin synthesis inhibitor PCPA (+) from E14.5 to E18.5. Note that PCPA treatment completely blocked the serotonin surge in MAO AB KO mice. The DA and NE level is very low at this stage and not affected by treatment of PCPA. B, Images showing serotonin immunoreactivity (green) in the frontal cortex of WT mice and MAO AB KO mice that had been treated with vehicle or PCPA during late embryonic development. Note that serotonin immunoreactivity was markedly elevated in the cortex of MAO AB mice compared with WT mice, and that PCPA treatment greatly decreased the serotonin immunoreactivity. C, Representative images of Nissl staining of a section through the frontal cortex of P1–P2 WT mice, and MAO AB KO mice that had been treated with either vehicle or PCPA during late embryonic development. The thickness reduction of SVZ in both telencephalon and striatum region is apparently restored by PCPA treatment in MAO AB KO mice. D, Quantification of SVZ thickness in P1–P2 WT mice and MAO AB KO mice that had been treated with vehicle or PCPA during embryonic development. Values for vehicle treated control mice (Con) and PCPA-treated mice are expressed as a percentage of the cortical thickness value for WT mice (mean and SEM; n = 4–5 mice). E, Images showing TBR2 immunoreactivity (green) and cell nuclei stained with PI (red) in brain sections from a P2 WT mouse and a P2 MAO AB KO mouse that had been treated with PCPA during late embryonic development. F, Values for numbers of TBR2+ cells in the SVZ of P2 WT mice and P2 MAO AB KO mouse that had been treated with PCPA during late embryonic development. Values are the mean and SEM (n = 4–5 mice).
Similar articles
- Early postnatal inhibition of serotonin synthesis results in long-term reductions of perseverative behaviors, but not aggression, in MAO A-deficient mice.
Bortolato M, Godar SC, Tambaro S, Li FG, Devoto P, Coba MP, Chen K, Shih JC. Bortolato M, et al. Neuropharmacology. 2013 Dec;75:223-32. doi: 10.1016/j.neuropharm.2013.07.003. Epub 2013 Jul 16. Neuropharmacology. 2013. PMID: 23871843 Free PMC article. - Altered gene expression in early postnatal monoamine oxidase A knockout mice.
Chen K, Kardys A, Chen Y, Flink S, Tabakoff B, Shih JC. Chen K, et al. Brain Res. 2017 Aug 15;1669:18-26. doi: 10.1016/j.brainres.2017.05.017. Epub 2017 May 20. Brain Res. 2017. PMID: 28535982 Free PMC article. - Laminin regulates postnatal oligodendrocyte production by promoting oligodendrocyte progenitor survival in the subventricular zone.
Relucio J, Menezes MJ, Miyagoe-Suzuki Y, Takeda S, Colognato H. Relucio J, et al. Glia. 2012 Oct;60(10):1451-67. doi: 10.1002/glia.22365. Epub 2012 Jun 17. Glia. 2012. PMID: 22706957 Free PMC article. - The progenitor cells of the embryonic telencephalon and the neonatal anterior subventricular zone differentially regulate their cell cycle.
Luskin MB, Coskun V. Luskin MB, et al. Chem Senses. 2002 Jul;27(6):577-80. doi: 10.1093/chemse/27.6.577. Chem Senses. 2002. PMID: 12142335 Free PMC article. Review. - Biochemical, behavioral, physiologic, and neurodevelopmental changes in mice deficient in monoamine oxidase A or B.
Holschneider DP, Chen K, Seif I, Shih JC. Holschneider DP, et al. Brain Res Bull. 2001 Nov 15;56(5):453-62. doi: 10.1016/s0361-9230(01)00613-x. Brain Res Bull. 2001. PMID: 11750790 Free PMC article. Review.
Cited by
- Does fetal growth restriction induce neuropathology within the developing brainstem?
Ahmadzadeh E, Polglase GR, Stojanovska V, Herlenius E, Walker DW, Miller SL, Allison BJ. Ahmadzadeh E, et al. J Physiol. 2023 Nov;601(21):4667-4689. doi: 10.1113/JP284191. Epub 2023 Aug 17. J Physiol. 2023. PMID: 37589339 Free PMC article. Review. - Constitutive Activity of Serotonin Receptor 6 Regulates Human Cerebral Organoids Formation and Depression-like Behaviors.
Wang Q, Dong X, Hu T, Qu C, Lu J, Zhou Y, Li J, Pei G. Wang Q, et al. Stem Cell Reports. 2021 Jan 12;16(1):75-88. doi: 10.1016/j.stemcr.2020.11.015. Epub 2020 Dec 23. Stem Cell Reports. 2021. PMID: 33357407 Free PMC article. - Neurotransmitters as Modulators of Neural Progenitor Cell Proliferation During Mammalian Neocortex Development.
Xing L, Huttner WB. Xing L, et al. Front Cell Dev Biol. 2020 May 26;8:391. doi: 10.3389/fcell.2020.00391. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32528958 Free PMC article. Review. - MAOA-VNTR genotype affects structural and functional connectivity in distributed brain networks.
Harneit A, Braun U, Geiger LS, Zang Z, Hakobjan M, van Donkelaar MMJ, Schweiger JI, Schwarz K, Gan G, Erk S, Heinz A, Romanczuk-Seiferth N, Witt S, Rietschel M, Walter H, Franke B, Meyer-Lindenberg A, Tost H. Harneit A, et al. Hum Brain Mapp. 2019 Dec 15;40(18):5202-5212. doi: 10.1002/hbm.24766. Epub 2019 Aug 23. Hum Brain Mapp. 2019. PMID: 31441562 Free PMC article. - In Utero Exposure to Citalopram Mitigates Maternal Stress Effects on Fetal Brain Development.
Velasquez JC, Zhao Q, Chan Y, Galindo LCM, Simasotchi C, Wu D, Hou Z, Herod SM, Oberlander TF, Gil S, Fournier T, Burd I, Andrews AM, Bonnin A. Velasquez JC, et al. ACS Chem Neurosci. 2019 Jul 17;10(7):3307-3317. doi: 10.1021/acschemneuro.9b00180. Epub 2019 Jun 24. ACS Chem Neurosci. 2019. PMID: 31184110 Free PMC article.
References
- Angevine JB, Jr, Bodian D, Coulombre AJ, Edds MV, Jr, Hamburger V, Jacobson M, Lyser KM, Prestige MC, Sidman RL, Varon S, Weiss PA. Embryonic vertebrate central nervous system: revised terminology. Anat Rec. 1970;166:257–261. - PubMed
- Banasr M, Hery M, Brezun JM, Daszuta A. Serotonin mediates oestrogen stimulation of cell proliferation in the adult dentate gyrus. Eur J Neurosci. 2001;14:1417–1424. - PubMed
- Banasr M, Hery M, Printemps R, Daszuta A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology. 2004;29:450–460. - PubMed
- Bauer S. Cytokine control of adult neural stem cells. Ann N Y Acad Sci. 2009;1153:48–56. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 MH39085/MH/NIMH NIH HHS/United States
- R01 MH039085/MH/NIMH NIH HHS/United States
- R37 MH039085/MH/NIMH NIH HHS/United States
- R01 MH039085-26/MH/NIMH NIH HHS/United States
- R37 MH39085/MH/NIMH NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases