Why so few drugs for Alzheimer's disease? Are methods failing drugs? - PubMed (original) (raw)
Review
Why so few drugs for Alzheimer's disease? Are methods failing drugs?
R E Becker et al. Curr Alzheimer Res. 2010 Nov.
Abstract
Recent studies of Alzheimer's disease (AD) and other neuropsychiatric drug developments raise questions whether failures of some drugs occur due to flaws in methods. In three case studies of recent AD drug development failures with phenserine, metrifonate, and tarenflurbil we identified methodological lapses able to account for the failures. Errors in complex systems such as drug developments are both almost inescapable due to human mistakes and most frequently hidden at the time of occurrence and thereafter. We propose preemptive error management as a preventive strategy to exclude or control error intrusions into neuropsychiatric drug developments. We illustrate the functions we anticipate for a preemptive error management preventive strategy with a checklist and identify the limitations of this aspect of the proposal with three drug examples. This strategy applies core scientific practices to insure the quality of data within the current context of AD drug development practices.
Figures
Fig. (1)
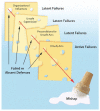
Swiss Cheese model of error [13]. As illustrated, during processes of planning and executing complex processes, investigators and sponsors allow lapses in defenses against mistakes (Active Failures or Unsafe Acts) opening the processes or outputs to errors. These lapses may be oversights or results from effective interventions at multiple levels being overcome by random shifts and realignments that allow, as the figure illustrates, unexpected errors and consequences.
Fig. (2)
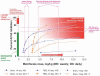
Relation between tarenflurbil action in mild to moderate Alzheimer's disease and maximum plasma concentrations of tarenflurbil (Cmax) in a randomized phase II CT trial [reprinted from [6], Figure 3C]. The straight line of the ascending improvements in the outcome measure at the highest dose tested, expressed as the Cmax, allow the possibility that more efficacy may occur at greater doses than those within the tested range that were selected for the Phase II CT. All of these tested doses were described as safe and well tolerated, suggesting that tolerability may not have been an issue in not selecting a higher dose.
Fig. (3)
Efficacy and Toxicity with Metrifonate (mg/kg/wk): Weekly Versus Daily Dosing Effects on Percent AChE Inhibition (Assessed in Red Blood Cells (RBC). Legend: These previously mainly unpublished data are from the author's (REB) laboratory and from [5, 15, 16]. These data are based on work completed by the author and the monographs [39, 40]. The distinction exemplified favors the weekly dosing both because the estimated replacement half-lives of acetylcholinesterase of 4 and 14 days [5] and recovery of other enzymes irreversibly inhibited by the organophosphate pro-drug metrifonate activation [5] are thought to allow recovery by new protein synthesis or activation which does not occur adequately with daily dosing.
Fig. (4)
Variance Sources and Consequences. Inter-site variance in the placebo data derived from the initial phase III CT of phenserine in mild to moderate AD (Axonyx AX-CL-06) (Data from Axonyx master file by permission of QR Pharma). The further variance around the mean values for each site (intra-site variance) is not shown. In light of the expected moderate improvement in ADAS-cog found for anticholinesterases in AD, a large variance in placebo data may over shadow and mask any drug related efficacy signal.
Similar articles
- Fire in the ashes: can failed Alzheimer's disease drugs succeed with second chances?
Becker RE, Greig NH. Becker RE, et al. Alzheimers Dement. 2013 Jan;9(1):50-7. doi: 10.1016/j.jalz.2012.01.007. Epub 2012 Mar 30. Alzheimers Dement. 2013. PMID: 22465172 Free PMC article. - The perils of Alzheimer's drug development.
Schneider LS, Lahiri DK. Schneider LS, et al. Curr Alzheimer Res. 2009 Feb;6(1):77-8. doi: 10.2174/156720509787313871. Curr Alzheimer Res. 2009. PMID: 19199878 Free PMC article. No abstract available. - Was phenserine a failure or were investigators mislead by methods?
Becker RE, Greig NH. Becker RE, et al. Curr Alzheimer Res. 2012 Dec;9(10):1174-81. doi: 10.2174/156720512804142912. Curr Alzheimer Res. 2012. PMID: 23227991 Free PMC article. - Alzheimer's disease drug development in 2008 and beyond: problems and opportunities.
Becker RE, Greig NH. Becker RE, et al. Curr Alzheimer Res. 2008 Aug;5(4):346-57. doi: 10.2174/156720508785132299. Curr Alzheimer Res. 2008. PMID: 18690832 Free PMC article. Review. - Alzheimer's disease drug development: old problems require new priorities.
Becker RE, Greig NH. Becker RE, et al. CNS Neurol Disord Drug Targets. 2008 Dec;7(6):499-511. doi: 10.2174/187152708787122950. CNS Neurol Disord Drug Targets. 2008. PMID: 19128207 Free PMC article. Review.
Cited by
- Interactions of cyclodextrins and their derivatives with toxic organophosphorus compounds.
Letort S, Balieu S, Erb W, Gouhier G, Estour F. Letort S, et al. Beilstein J Org Chem. 2016 Feb 5;12:204-28. doi: 10.3762/bjoc.12.23. eCollection 2016. Beilstein J Org Chem. 2016. PMID: 26977180 Free PMC article. Review. - Does traumatic brain injury hold the key to the Alzheimer's disease puzzle?
Becker RE, Kapogiannis D, Greig NH. Becker RE, et al. Alzheimers Dement. 2018 Apr;14(4):431-443. doi: 10.1016/j.jalz.2017.11.007. Epub 2017 Dec 12. Alzheimers Dement. 2018. PMID: 29245000 Free PMC article. Review. - Alzheimer disease therapy--moving from amyloid-β to tau.
Giacobini E, Gold G. Giacobini E, et al. Nat Rev Neurol. 2013 Dec;9(12):677-86. doi: 10.1038/nrneurol.2013.223. Epub 2013 Nov 12. Nat Rev Neurol. 2013. PMID: 24217510 Review. - J-147 a Novel Hydrazide Lead Compound to Treat Neurodegeneration: CeeTox™ Safety and Genotoxicity Analysis.
Lapchak PA, Bombien R, Rajput PS. Lapchak PA, et al. J Neurol Neurophysiol. 2013 Aug;4(3):158. doi: 10.4172/2155-9562.1000158. J Neurol Neurophysiol. 2013. PMID: 25364619 Free PMC article. - Phenserine efficacy in Alzheimer's disease.
Winblad B, Giacobini E, Frölich L, Friedhoff LT, Bruinsma G, Becker RE, Greig NH. Winblad B, et al. J Alzheimers Dis. 2010;22(4):1201-8. doi: 10.3233/JAD-2010-101311. J Alzheimers Dis. 2010. PMID: 20930279 Free PMC article. Clinical Trial.
References
- Aisen PS, Gauthier S, Vellas B, Briand R, Saumier D, Laurin J, et al. Alzhemed: a potential treatment for Alzheimer's disease. Curr Alz Res. 2007;4:473–478. - PubMed
- Alzforum FDA deems U.S. Alzhemed trial results inconclusive. Viewed April 15, 2010: http://www.alzforum.org/new/detail.asp?id=1647.
- Gauthier S, Aisen PS, Ferris SH, Saumier D, Duong A, Haine D, et al. Effect of tramiprosate in patients with mild-to-moderate Alzheimer's disease: exploratory analyses of the MRI sub-group of the Alphase study. J Nutr Health Aging. 2009;13:550–557. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical