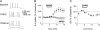Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons - PubMed (original) (raw)
Memory for fear extinction requires mGluR5-mediated activation of infralimbic neurons
Darah E Fontanez-Nuin et al. Cereb Cortex. 2011 Mar.
Abstract
Consolidation of fear extinction involves enhancement of N-methyl D aspartate (NMDA) receptor-dependent bursting in the infralimbic region (IL) of the medial prefrontal cortex (mPFC). Previous studies have shown that systemic blockade of metabotropic glutamate receptor type 5 (mGluR5) reduces bursting in the mPFC and mGluR5 agonists enhance NMDA receptor currents in vitro, suggesting that mGluR5 activation in IL may contribute to fear extinction. In the current study, rats injected with the mGluR5 antagonist 2-methyl-6-(phenylethyl)-pyridine (MPEP) systemically, or intra-IL, prior to extinction exhibited normal within-session extinction, but were impaired in their ability to recall extinction the following day. To directly determine whether mGluR5 stimulation enhances the burst firing of IL neurons, we used patch-clamp electrophysiology in prefrontal slices. The mGluR5 agonist, (RS)-2-chloro-5-hydroxyphenylglycine (CHPG), increased intrinsic bursting in IL neurons. Increased bursting was correlated with a reduction in the slow after hyperpolarizing potential and was prevented by coapplication of MPEP. CHPG did not increase NMDA currents, suggesting that an NMDA receptor-independent enhancement of IL bursting via stimulation of mGluR5 receptors contributes to fear extinction. Therefore, the mGluR5 receptor could be a suitable target for pharmacological adjuncts to extinction-based therapies for anxiety disorders.
Figures
Figure 1.
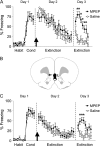
Systemic or IL blockade of mGluR5 impairs recall of fear extinction. (A) Percent freezing to the tone for saline-injected rats (n = 10) and rats injected intraperitoneally with 10 mg/kg MPEP (n = 12). MPEP-treated rats showed more freezing on day 3; *P < 0.001. (B) A coronal drawing (bregma, 3.20 mm) showing placements of injector tips for all rats that received IL infusions. (C) Percent freezing to the tone for saline-infused rats (n = 12) and rats infused with 1.5 μg in 0.5-μL MPEP into IL (n = 16); *P < 0.01. Arrow indicates the time of the injection.
Figure 2.
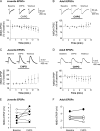
Activation of mGluR5 increases the intrinsic excitability of IL pyramidal neurons in juvenile rats. (A) Traces showing the number of spikes evoked by a current pulse during baseline, perfusion with the mGluR5 agonist, CHPG (500 μM), and washout in an IL neuron from a P19 rat. (B) Time course showing that CHPG (bar) increased the number of spikes (n = 8). The increase in spikes was blocked by the mGluR5 antagonist MPEP (100 μM, unfilled circles). (C) Time course demonstrating that CHPG also decreased the first interspike interval.
Figure 3.
Activation of mGluR5 decreases the sAHP. (A) _A_verage traces of the sAHP evoked by an 800-ms depolarizing pulse during baseline (black) and CHPG (red). Traces are truncated to emphasize the AHP. (B–C) Time course and bar graph showing that CHPG perfusion (500 μM; red bar) decreased the sAHP. MPEP prevented the CHPG-mediated reduction in the sAHP. (D) Average traces of AHP currents evoked by 800-ms step from −50 to 0 mV. CHPG blocked the slower component leaving a faster component that was blocked by apamin (100 nM). (E) This current was also blocked by norepinephrine (NE, 100 μM), a known blocker of the _I_sAHP. (F) Bar graph summarizing the effects of CHPG and apamin on the AHP currents. *P < 0.05 compared with baseline. #P < 0.05 compared with CHPG.
Figure 4.
CHPG also increases the intrinsic excitability of IL pyramidal neurons from adult rats_._ (A) Traces showing the number of spikes evoked by a current pulse during baseline, perfusion with CHPG (500 μM), and washout in a pyramidal neuron from a P60 rat. (B) Time course demonstrating that CHPG (bar, n = 7) increased the number of spikes. (C) Time course showing that CHPG reduced the first interspike interval. (D) Time course showing that CHPG reduced the sAHP.
Figure 5.
mGluR5 effects on NMDA receptor–mediated EPSCs or EPSPs in IL slices from juvenile and adult rats. (A,B) _T_ime courses showing NMDA EPSC amplitudes during baseline, application of CHPG (500 μM; bar), and washout. Traces show average EPSCs in IL neurons from a P28 (A) and a P60 (B) rat. (C,D) Time courses showing NMDA EPSP amplitudes during baseline, application of CHPG, and washout. Traces show average EPSPs in IL neurons from a P28 (C) and a P60 (D) rat. (E,F) NMDA EPSP amplitudes of all neurons from the juvenile and adult rats during baseline and perfusion of CHPG. Black filled circles indicate EPSPs that were increased by CHPG (P < 0.01).
Similar articles
- Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons.
Sepulveda-Orengo MT, Lopez AV, Soler-Cedeño O, Porter JT. Sepulveda-Orengo MT, et al. J Neurosci. 2013 Apr 24;33(17):7184-93. doi: 10.1523/JNEUROSCI.5198-12.2013. J Neurosci. 2013. PMID: 23616528 Free PMC article. - M-type potassium channels modulate the intrinsic excitability of infralimbic neurons and regulate fear expression and extinction.
Santini E, Porter JT. Santini E, et al. J Neurosci. 2010 Sep 15;30(37):12379-86. doi: 10.1523/JNEUROSCI.1295-10.2010. J Neurosci. 2010. PMID: 20844133 Free PMC article. - Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction.
Santini E, Sepulveda-Orengo M, Porter JT. Santini E, et al. Neuropsychopharmacology. 2012 Aug;37(9):2047-56. doi: 10.1038/npp.2012.52. Epub 2012 Apr 18. Neuropsychopharmacology. 2012. PMID: 22510723 Free PMC article. - Pharmacological interference with metabotropic glutamate receptor subtype 7 but not subtype 5 differentially affects within- and between-session extinction of Pavlovian conditioned fear.
Toth I, Dietz M, Peterlik D, Huber SE, Fendt M, Neumann ID, Flor PJ, Slattery DA. Toth I, et al. Neuropharmacology. 2012 Mar;62(4):1619-26. doi: 10.1016/j.neuropharm.2011.10.021. Epub 2011 Nov 10. Neuropharmacology. 2012. PMID: 22079159 - Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons.
Santini E, Quirk GJ, Porter JT. Santini E, et al. J Neurosci. 2008 Apr 9;28(15):4028-36. doi: 10.1523/JNEUROSCI.2623-07.2008. J Neurosci. 2008. PMID: 18400902 Free PMC article.
Cited by
- Fear extinction induces mGluR5-mediated synaptic and intrinsic plasticity in infralimbic neurons.
Sepulveda-Orengo MT, Lopez AV, Soler-Cedeño O, Porter JT. Sepulveda-Orengo MT, et al. J Neurosci. 2013 Apr 24;33(17):7184-93. doi: 10.1523/JNEUROSCI.5198-12.2013. J Neurosci. 2013. PMID: 23616528 Free PMC article. - mGluR5 Positive and Negative Allosteric Modulators Differentially Affect Dendritic Spine Density and Morphology in the Prefrontal Cortex.
LaCrosse AL, Taylor SB, Nemirovsky NE, Gass JT, Olive MF. LaCrosse AL, et al. CNS Neurol Disord Drug Targets. 2015;14(4):476-85. doi: 10.2174/1871527314666150429112849. CNS Neurol Disord Drug Targets. 2015. PMID: 25921744 Free PMC article. - The Plasticity of Extinction: Contribution of the Prefrontal Cortex in Treating Addiction through Inhibitory Learning.
Gass JT, Chandler LJ. Gass JT, et al. Front Psychiatry. 2013 May 30;4:46. doi: 10.3389/fpsyt.2013.00046. eCollection 2013. Front Psychiatry. 2013. PMID: 23750137 Free PMC article. - Persisting Verbal Memory Encoding and Recall Deficiency after mGluR5 Autoantibody-Mediated Encephalitis.
Hansen N, Rentzsch K, Hirschel S, Wiltfang J, Schott BH, Malchow B, Bartels C. Hansen N, et al. Brain Sci. 2023 Oct 31;13(11):1537. doi: 10.3390/brainsci13111537. Brain Sci. 2023. PMID: 38002497 Free PMC article. - Differential Role of mGluR5 in Cognitive Processes in Posttraumatic Stress Disorder and Major Depression.
Esterlis I, DeBonee S, Cool R, Holmes S, Baldassari SR, Maruff P, Pietrzak RH, Davis MT. Esterlis I, et al. Chronic Stress (Thousand Oaks). 2022 Aug 4;6:24705470221105804. doi: 10.1177/24705470221105804. eCollection 2022 Jan-Dec. Chronic Stress (Thousand Oaks). 2022. PMID: 35958037 Free PMC article.
References
- Anderson JJ, Rao SP, Rowe B, Giracello DR, Holtz G, Chapman DF, Tehrani L, Bradbury MJ, Cosford NDP, Varney MA. [3H]Methoxymethyl-3-[(2-methyl-1,3-thiazol-4-yl)ethynyl]pyridine binding to metabotropic glutamate receptor subtype 5 in rodent brain: in vitro and in vivo characterization. J Pharmacol Exp Ther. 2002;303:1044–1051. - PubMed
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther. 2007;45:2019–2033. - PubMed
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31-GM075489/GM/NIGMS NIH HHS/United States
- R01-MH058883/MH/NIMH NIH HHS/United States
- R01 MH058883/MH/NIMH NIH HHS/United States
- R01 MH081975/MH/NIMH NIH HHS/United States
- F31 GM075489/GM/NIGMS NIH HHS/United States
- R01-MH081975/MH/NIMH NIH HHS/United States
- G12 RR003050/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical