Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons - PubMed (original) (raw)
Review
Cocaine-induced homeostatic regulation and dysregulation of nucleus accumbens neurons
Yanhua H Huang et al. Behav Brain Res. 2011.
Abstract
Homeostatic response is an endowed self-correcting/maintaining property for living units, ranging from subcellular domains, single cells, and organs to the whole organism. Homeostatic responses maintain stable function through the ever-changing internal and external environments. In central neurons, several forms of homeostatic regulation have been identified, all of which tend to stabilize the functional output of neurons toward their prior "set-point." Medium spiny neurons (MSNs) within the forebrain region the nucleus accumbens (NAc) play a central role in gating/regulating emotional and motivational behaviors including craving and seeking drugs of abuse. Exposure to highly salient stimuli such as cocaine administration not only acutely activates a certain population of NAc MSNs, but also induces long-lasting changes in these neurons. It is these long-lasting cellular alterations that are speculated to mediate the increasingly strong cocaine-craving and cocaine-seeking behaviors. Why do the potentially powerful homeostatic mechanisms fail to correct or compensate for these drug-induced maladaptations in neurons? Based on recent experimental results, this review proposes a hypothesis of homeostatic dysregulation induced by exposure to cocaine. Specifically, we hypothesize that exposure to cocaine generates false molecular signals which misleads the homeostatic regulation process, resulting in maladaptive changes in NAc MSNs. Thus, many molecular and cellular alterations observed in the addicted brain may indeed result from homeostatic dysregulation. This review is among the first to introduce the concept of homeostatic neuroplasticity to understanding the molecular and cellular maladaptations following exposure to drugs of abuse.
Copyright © 2010 Elsevier B.V. All rights reserved.
Figures
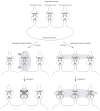
Figure 1
Using synaptic plasticity as an example, the diagram shows the differences between regulated and homeostatic plasticity. A A postsynaptic neuron receives multiple presynaptic inputs (1, 2, and 3). These presynaptic terminals may project from different neurons and thus may also represent synapses from different pathways. B Regulated synaptic plasticity exhibits two defining properties, contingency and specificity. A successful induction of regulated synaptic plasticity requires a contingent activation of pre- and postsynaptic terminals (diagramed as action potential firing at both presynaptic and postsynaptic terminals at synapse 2; affected synapse is in shade), and is only expressed in the affected pathway (e.g., only synapse 2 is potentiated by enhanced postsynaptic responsiveness). Other synapses (1 and 3), although located on the same postsynaptic neuron, are not altered because they are not within the contingently activated pathway. C Homeostatic synaptic plasticity is induced and expressed “globally”. Chronic decrease of postsynaptic receptor sensitivity induces a global increase in presynaptic release such that the action potential firing of postsynaptic neurons is restored.
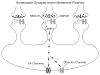
Figure 2
A schematic view of homeostatic synapse-driven membrane plasticity in NAc neurons. On the postsynaptic membrane, AMPARs mediate most of postsynaptic current, and NMDARs are also activated during synaptic transmission. The activity of synaptic NMDARs is positively correlated with the activity level of excitatory synapses. As such, the constitutively active NMDAR-coupled intracellular signaling (e.g., the CaMKII-mediated signaling) can be up- or down-regulated accordingly on a real-time base, which in turn modulates ion channels (e.g., SK channels) located on the somatic membrane.
Figure 3
Summary of synaptic and membrane alterations in NAc neurons following non-contingent exposure to cocaine. A typical non-contingent cocaine procedure is to treat the animal with 5-day i.p. injections of cocaine (15–20 mg/kg/day), followed by different withdrawal periods. An important synaptic alteration in NAc neurons during the late phase of cocaine exposure and short-term withdrawal is the appearance of silent synapses enriched in NR2B-containing NMDARs. These silent synapses decrease over time during long-term withdrawal. Synaptic/surface AMPARs in NAc neurons are altered minimally if any (i.e., a slight decrease) during short-term withdrawal, but are greatly up-regulated during long-term withdrawal. The intrinsic membrane excitability of NAc neurons is decreased throughout short- and long-term withdrawal. Based on the timing of these cocaine-induced synaptic and membrane alterations, we hypothesize that these cellular alterations are homeostatically linked. For example, although synaptic AMPARs are not up-regulated during short-term withdrawal, the increased NMDAR-mediated activity may create a false signal of increased synaptic strength to trigger hSMP, resulting in observed decrease in membrane excitability (?1). Furthermore, newly generated silent synapses provide extra synaptic lots that may facilitate synaptic recruitment of new AMPARs during long-tem withdrawal (?2). The synaptic recruitment of AMPARs during long-term withdrawal may result from regulated or homeostatic plasticity. For homeostatic plasticity, a potential mechanism is that the decreased membrane excitability may trigger another round of membrane-to-synapse homeostatic response, resulting in an increase in excitatory synaptic strength in NAc neurons (?3).
Similar articles
- Caveolin-1 regulates medium spiny neuron structural and functional plasticity.
Eisinger KRT, Chapp AD, Swanson SP, Tam D, Lopresti NM, Larson EB, Thomas MJ, Lanier LM, Mermelstein PG. Eisinger KRT, et al. Psychopharmacology (Berl). 2020 Sep;237(9):2673-2684. doi: 10.1007/s00213-020-05564-2. Epub 2020 Jun 2. Psychopharmacology (Berl). 2020. PMID: 32488350 Free PMC article. - Cascades of Homeostatic Dysregulation Promote Incubation of Cocaine Craving.
Wang J, Ishikawa M, Yang Y, Otaka M, Kim JY, Gardner GR, Stefanik MT, Milovanovic M, Huang YH, Hell JW, Wolf ME, Schlüter OM, Dong Y. Wang J, et al. J Neurosci. 2018 May 2;38(18):4316-4328. doi: 10.1523/JNEUROSCI.3291-17.2018. Epub 2018 Apr 6. J Neurosci. 2018. PMID: 29626166 Free PMC article. - Homeostatic synapse-driven membrane plasticity in nucleus accumbens neurons.
Ishikawa M, Mu P, Moyer JT, Wolf JA, Quock RM, Davies NM, Hu XT, Schlüter OM, Dong Y. Ishikawa M, et al. J Neurosci. 2009 May 6;29(18):5820-31. doi: 10.1523/JNEUROSCI.5703-08.2009. J Neurosci. 2009. PMID: 19420249 Free PMC article. - Cocaine-induced metaplasticity in the nucleus accumbens: silent synapse and beyond.
Lee BR, Dong Y. Lee BR, et al. Neuropharmacology. 2011 Dec;61(7):1060-9. doi: 10.1016/j.neuropharm.2010.12.033. Epub 2011 Jan 11. Neuropharmacology. 2011. PMID: 21232547 Free PMC article. Review. - Regulation of AMPA receptor trafficking in the nucleus accumbens by dopamine and cocaine.
Wolf ME. Wolf ME. Neurotox Res. 2010 Nov;18(3-4):393-409. doi: 10.1007/s12640-010-9176-0. Epub 2010 Apr 2. Neurotox Res. 2010. PMID: 20361291 Free PMC article. Review.
Cited by
- Subregional, dendritic compartment, and spine subtype specificity in cocaine regulation of dendritic spines in the nucleus accumbens.
Dumitriu D, Laplant Q, Grossman YS, Dias C, Janssen WG, Russo SJ, Morrison JH, Nestler EJ. Dumitriu D, et al. J Neurosci. 2012 May 16;32(20):6957-66. doi: 10.1523/JNEUROSCI.5718-11.2012. J Neurosci. 2012. PMID: 22593064 Free PMC article. - Caveolin-1 regulates medium spiny neuron structural and functional plasticity.
Eisinger KRT, Chapp AD, Swanson SP, Tam D, Lopresti NM, Larson EB, Thomas MJ, Lanier LM, Mermelstein PG. Eisinger KRT, et al. Psychopharmacology (Berl). 2020 Sep;237(9):2673-2684. doi: 10.1007/s00213-020-05564-2. Epub 2020 Jun 2. Psychopharmacology (Berl). 2020. PMID: 32488350 Free PMC article. - Sexually dimorphic prelimbic cortex mechanisms play a role in alcohol dependence: protection by endostatin.
Avchalumov Y, Kreisler AD, Xing N, Shayan AA, Bharadwaj T, Watson JR, Sibley B, Somkuwar SS, Trenet W, Olia S, Piña-Crespo JC, Roberto M, Mandyam CD. Avchalumov Y, et al. Neuropsychopharmacology. 2021 Oct;46(11):1937-1949. doi: 10.1038/s41386-021-01075-6. Epub 2021 Jul 12. Neuropsychopharmacology. 2021. PMID: 34253856 Free PMC article. - GABAergic neurons in nucleus accumbens are correlated to resilience and vulnerability to chronic stress for major depression.
Zhu Z, Wang G, Ma K, Cui S, Wang JH. Zhu Z, et al. Oncotarget. 2017 May 30;8(22):35933-35945. doi: 10.18632/oncotarget.16411. Oncotarget. 2017. PMID: 28415589 Free PMC article. - Amyloid-β oligomers in the nucleus accumbens decrease motivation via insertion of calcium-permeable AMPA receptors.
Guo C, Wen D, Zhang Y, Mustaklem R, Mustaklem B, Zhou M, Ma T, Ma YY. Guo C, et al. Mol Psychiatry. 2022 Apr;27(4):2146-2157. doi: 10.1038/s41380-022-01459-0. Epub 2022 Feb 2. Mol Psychiatry. 2022. PMID: 35105968 Free PMC article.
References
- Anderson SM, Famous KR, Sadri-Vakili G, Kumaresan V, Schmidt HD, Bass CE, Terwilliger EF, Cha JH, Pierce RC. CaMKII: a biochemical bridge linking accumbens dopamine and glutamate systems in cocaine seeking. Nat Neurosci. 2008;11:344–53. - PubMed
- Baker DA, McFarland K, Lake RW, Shen H, Tang XC, Toda S, Kalivas PW. Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci. 2003;6:743–9. - PubMed
- Barria A, Malinow R. NMDA receptor subunit composition controls synaptic plasticity by regulating binding to CaMKII. Neuron. 2005;48:289–301. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DA023206/DA/NIDA NIH HHS/United States
- R01 DA023206/DA/NIDA NIH HHS/United States
- DA023206/DA/NIDA NIH HHS/United States
- K99 DA029565/DA/NIDA NIH HHS/United States
- R01 DA023206-03/DA/NIDA NIH HHS/United States
LinkOut - more resources
Full Text Sources