Presence of a putative tumor-initiating progenitor cell population predicts poor prognosis in smokers with non-small cell lung cancer - PubMed (original) (raw)
. 2010 Aug 15;70(16):6639-48.
doi: 10.1158/0008-5472.CAN-10-0455.
Vei Mah, Derek W Nickerson, Jennifer L Gilbert, Vi Luan Ha, Ahmed E Hegab, Steve Horvath, Mohammad Alavi, Erin L Maresh, David Chia, Adam C Gower, Marc E Lenburg, Avrum Spira, Luisa M Solis, Ignacio I Wistuba, Tonya C Walser, William D Wallace, Steven M Dubinett, Lee Goodglick, Brigitte N Gomperts
Affiliations
- PMID: 20710044
- PMCID: PMC2924777
- DOI: 10.1158/0008-5472.CAN-10-0455
Presence of a putative tumor-initiating progenitor cell population predicts poor prognosis in smokers with non-small cell lung cancer
Aik T Ooi et al. Cancer Res. 2010.
Abstract
Smoking is the most important known risk factor for the development of lung cancer. Tobacco exposure results in chronic inflammation, tissue injury, and repair. A recent hypothesis argues for a stem/progenitor cell involved in airway epithelial repair that may be a tumor-initiating cell in lung cancer and which may be associated with recurrence and metastasis. We used immunostaining, quantitative real-time PCR, Western blots, and lung cancer tissue microarrays to identify subpopulations of airway epithelial stem/progenitor cells under steady-state conditions, normal repair, aberrant repair with premalignant lesions and lung cancer, and their correlation with injury and prognosis. We identified a population of keratin 14 (K14)-expressing progenitor epithelial cells that was involved in repair after injury. Dysregulated repair resulted in the persistence of K14+ cells in the airway epithelium in potentially premalignant lesions. The presence of K14+ progenitor airway epithelial cells in NSCLC predicted a poor prognosis, and this predictive value was strongest in smokers, in which it also correlated with metastasis. This suggests that reparative K14+ progenitor cells may be tumor-initiating cells in this subgroup of smokers with NSCLC.
(c)2010 AACR.
Conflict of interest statement
There are no conflicts of interest.
Figures
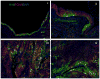
Figure 1. K14 and K5-expressing progenitor cell populations in the airway epithelium at steady state and during repair
A, B. Representative sections of immunofluorescent staining identifies cells in the submucosal glands and submucosal gland duct that express K14 (Alexa fluor 488, green) and K5 (Cy3, red). Basal cells of the pseudostratified columnar airway epithelium express K5 but do not express K14. A) is representative of immunostaining seen in mice (scale bar = 20μm), B) is representative of staining in humans (scale bar = 100μm). H&E stained representative sections are included to demonstrate the anatomy of the pseudostratified columnar airway epithelium (arrow), the submucosal glands (dotted arrow) and submucosal gland ducts (dashed arrow).
Figure 2
Figure 2A. Representative sections of immunofluorescent staining of K14 (Alexa fluor 488, green) and K5 (Cy3, red) expressing cells in the mouse tracheal airway epithelium after hypoxic-ischemic injury from tracheal transplantation. i. K14 and K5-expressing cells are seen in the submucosal glands, submucosal gland ducts and repairing surface airway epithelium. ii. K14 and K5-expressing cells are seen on the repairing surface airway epithelium. iii. K14 and K5-expressing cells are seen in a hyperplastic area of repairing surface airway epithelium but in areas of pseudostratified columnar epithelium K5 expression is present in the basal cells but K14 expression is absent. iv. Repaired pseudostratified columnar epithelium with K5 expression in the basal cells and absence of K14 expression. Corresponding H&E sections are included to demonstrate the histopathology of the repairing airway. 2B. Representative sections of immunofluorescent staining of K14 (Alexa fluor 488, green) and K5 (Cy3, red) expressing cells in repairing airway epithelial human tissue from smokers with reserve cell hyperplasia and squamous metaplasia. K5+K14- basal cells are seen in normal airway epithelium (red arrow). A few K14+K5+ few basal cells are also present (yellow arrow). K14+K5+ cells are seen in an area of reserve cell hyperplasia and in squamous metaplasia (green arrows). H&E staining of the section demonstrates the areas of normal pseudostratified columnar epithelium (arrows), reserve cell hyperplasia (dotted arrow), and squamous metaplasia (dashed arrow), (scale bar = 20μm). 2C. Representative sections of immunofluorescent staining of K14 (Alexa fluor 488, green) and K5 (Cy3, red) expressing cells in repairing airway epithelial human tissue from smokers with dysplasia and carcinoma in situ lesions. K14+K5+ cells are seen in areas of moderate dysplasia (green arrows) and carcinoma in situ (severe dysplasia)(green dashed arrow). H&E staining of the section demonstrates the areas of moderate dysplasia (arrows) and carcinoma in situ (severe dysplasia) (dashed arrow), (scale bar = 20μm).
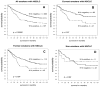
Figure 3. Kaplan Meier Survival curves showing that K14 expression in NSCLC correlates with poor prognosis
A. Analysis of the UCLA TMA revealed that patients with NSCLC that expressed K14 had a significantly worse prognosis than patients with NSCLC in which K14 was below the level of detection (P=0.004, hazard ratio = 1.58). B. Analysis of the M.D. Anderson TMA also showed that patients with NSCLC that expressed K14 had a worse prognosis than patients with NSCLC in which K14 was below the level of detection (P=0.003, hazard ration = 1.60).
Figure 4. Kaplan Meier Survival curves from the UCLA TMA showing that the poor prognosis related to K14-expressing NSCLC tumors correlated with smoking
A. In all smokers (current and former) K14 positivity in NSCLC tumors had the highest predictive value of death from NSCLC (P=0.0009, hazard ratio = 1.77, n = 332). B. The predictive value of K14 expressing NSCLC tumors in individuals who were current smokers (P=0.01, hazard ratio = 2.11, n = 124). C. K14 positivity was still somewhat predictive of death due to disease in former smokers as well (P=0.04, hazard ratio = 1.68, n = 157). D. In never smokers, the presence of K14+ cells had no predictive value for outcome (P=0.93, hazard ratio = 0.95, n=53).
Figure 5. Dual immunofluorescent staining of human premalignant lesions and tumors to assess populations of proliferating cells that also express K14
i. and ii. Dual immunofluorescent staining of premalignant lesions for K14 and PCNA. In premalignant lesions we found that 57.8% ± 5.1% of K14+ cells also expressed PCNA. iii and iv. Dual immunofluorescent staining of tissue from SCC for K14 and PCNA. In SCC we found that 67.3% ± 7.3% of K14+ cells also expressed PCNA
Similar articles
- Depressing time: Waiting, melancholia, and the psychoanalytic practice of care.
Salisbury L, Baraitser L. Salisbury L, et al. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. In: Kirtsoglou E, Simpson B, editors. The Time of Anthropology: Studies of Contemporary Chronopolitics. Abingdon: Routledge; 2020. Chapter 5. PMID: 36137063 Free Books & Documents. Review. - Genedrive kit for detecting single nucleotide polymorphism m.1555A>G in neonates and their mothers: a systematic review and cost-effectiveness analysis.
Shabaninejad H, Kenny RP, Robinson T, Stoniute A, O'Keefe H, Still M, Thornton C, Pearson F, Beyer F, Meader N. Shabaninejad H, et al. Health Technol Assess. 2024 Oct;28(75):1-75. doi: 10.3310/TGAC4201. Health Technol Assess. 2024. PMID: 39487741 Free PMC article. - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2020 Aug 27;8(8):CD007807. doi: 10.1002/14651858.CD007807.pub4. Cochrane Database Syst Rev. 2020. PMID: 32851663 Free PMC article. - Comparison of Two Modern Survival Prediction Tools, SORG-MLA and METSSS, in Patients With Symptomatic Long-bone Metastases Who Underwent Local Treatment With Surgery Followed by Radiotherapy and With Radiotherapy Alone.
Lee CC, Chen CW, Yen HK, Lin YP, Lai CY, Wang JL, Groot OQ, Janssen SJ, Schwab JH, Hsu FM, Lin WH. Lee CC, et al. Clin Orthop Relat Res. 2024 Dec 1;482(12):2193-2208. doi: 10.1097/CORR.0000000000003185. Epub 2024 Jul 23. Clin Orthop Relat Res. 2024. PMID: 39051924 - Antioxidants for female subfertility.
Showell MG, Mackenzie-Proctor R, Jordan V, Hart RJ. Showell MG, et al. Cochrane Database Syst Rev. 2017 Jul 28;7(7):CD007807. doi: 10.1002/14651858.CD007807.pub3. Cochrane Database Syst Rev. 2017. PMID: 28752910 Free PMC article. Updated. Review.
Cited by
- A lung cancer molecular prognostic test ready for prime time.
Xie Y, Minna JD. Xie Y, et al. Lancet. 2012 Mar 3;379(9818):785-7. doi: 10.1016/S0140-6736(12)60154-8. Lancet. 2012. PMID: 22386017 Free PMC article. No abstract available. - High expression of AGR2 in lung cancer is predictive of poor survival.
Alavi M, Mah V, Maresh EL, Bagryanova L, Horvath S, Chia D, Goodglick L, Liu AY. Alavi M, et al. BMC Cancer. 2015 Oct 6;15:655. doi: 10.1186/s12885-015-1658-2. BMC Cancer. 2015. PMID: 26445321 Free PMC article. - Identification of Six Prognostic Genes in EGFR-Mutant Lung Adenocarcinoma Using Structure Network Algorithms.
Zhang H, Lu D, Li Q, Lu F, Zhang J, Wang Z, Lu X, Wang J. Zhang H, et al. Front Genet. 2021 Nov 16;12:755245. doi: 10.3389/fgene.2021.755245. eCollection 2021. Front Genet. 2021. PMID: 34868228 Free PMC article. - CD117 expression is a predictive marker for poor prognosis in patients with non-small cell lung cancer.
Sakabe T, Azumi J, Haruki T, Umekita Y, Nakamura H, Shiota G. Sakabe T, et al. Oncol Lett. 2017 May;13(5):3703-3708. doi: 10.3892/ol.2017.5925. Epub 2017 Mar 27. Oncol Lett. 2017. PMID: 28521472 Free PMC article. - Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats.
Inoue T, Sugiyama M, Hattori H, Wakita H, Wakabayashi T, Ueda M. Inoue T, et al. Tissue Eng Part A. 2013 Jan;19(1-2):24-9. doi: 10.1089/ten.TEA.2011.0385. Epub 2012 Oct 10. Tissue Eng Part A. 2013. PMID: 22839964 Free PMC article.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. - PubMed
- van Klaveren RJ, van’t Westeinde SC, de Hoop BJ, Hoogsteden HC. Stem cells and the natural history of lung cancer: implications for lung cancer screening. Clin Cancer Res. 2009;15:2215–8. - PubMed
- McDonald SA, Graham TA, Schier S, Wright NA, Alison MR. Stem cells and solidcancers. Virchows Arch. 2009;455:1–13. - PubMed
- Ailles LE, Weissman IL. Cancer stem cells in solid tumors. Curr Opin Biotechnol. 2007;18:460–6. - PubMed
- Boman BM, Wicha MS. Cancer stem cells: a step toward the cure. J Clin Oncol. 2008;26:2795–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA86366/CA/NCI NIH HHS/United States
- K08 HL074229-01A2/HL/NHLBI NIH HHS/United States
- K08 HL074229-04/HL/NHLBI NIH HHS/United States
- K08 HL074229/HL/NHLBI NIH HHS/United States
- K08 HL074229-05/HL/NHLBI NIH HHS/United States
- K08 HL074229-03/HL/NHLBI NIH HHS/United States
- U24 CA086366/CA/NCI NIH HHS/United States
- K08 HL074229-02/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous