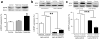MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212 - PubMed (original) (raw)
MeCP2 controls BDNF expression and cocaine intake through homeostatic interactions with microRNA-212
Heh-In Im et al. Nat Neurosci. 2010 Sep.
Abstract
The X-linked transcriptional repressor methyl CpG binding protein 2 (MeCP2), known for its role in the neurodevelopmental disorder Rett syndrome, is emerging as an important regulator of neuroplasticity in postmitotic neurons. Cocaine addiction is commonly viewed as a disorder of neuroplasticity, but the potential involvement of MeCP2 has not been explored. Here we identify a key role for MeCP2 in the dorsal striatum in the escalating cocaine intake seen in rats with extended access to the drug, a process that mimics the increasingly uncontrolled cocaine use seen in addicted humans. MeCP2 regulates cocaine intake through homeostatic interactions with microRNA-212 (miR-212) to control the effects of cocaine on striatal brain-derived neurotrophic factor (BDNF) levels. These data suggest that homeostatic interactions between MeCP2 and miR-212 in dorsal striatum may be important in regulating vulnerability to cocaine addiction.
Figures
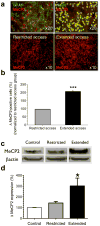
Figure 1. Increased striatal MeCP2 expression in extended access rats
(a) Immunochemical detection of MeCP2 in the dorsal striatum of drug-naïve rats. Left upper panel, MeCP2 (shown in red) rarely co-localized with glial fibrillary acidic protein (GFAP; shown in green), a marker for astrocytes. Right upper panel, MeCP2 (in red) was almost exclusively co-localized with the neuronal nuclear marker NeuN (shown in green). There was an increase in the number of MeCP2-positive cells in the dorsal striatum of rats with extended cocaine access (lower right panel) compared to rats with restricted access (lower left panel). (b) Relative numbers of MeCP2-positive cells shown in the lower left and right panels above. ***P<0.001, t-test. (c) Representative immunoblot demonstrating that MeCP2 expression is increased in the dorsal striatum of rats with extended cocaine access compared with restricted access or drug-naïve control rats. (d) Relative amounts of MeCP2 in dorsal striatum was quantified by densitometry. *P<0.05 compared with control, post-hoc comparison after significant one-way ANOVA. In all cases, _n_=6 rats per group, and error bars are given as s.e.m.
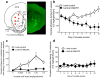
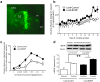
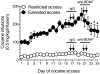
Figure 2. Dissociable effects of MeCP2 knockdown on cocaine intake
(a) The left panel is a graphical representation of the dorsal striatum and surrounding brain structures. Red circles represent the locations at which the Lenti-sh-MeCP2 virus infusions were targeted. The right panel is a representative immunochemistry staining from the brain of a Lenti-sh-MeCP2 rat. Green staining is GFP from virus; CTX, cortex; cc, corpus callosum; LV, lateral ventricle; DS, dorsal striatum; VS, ventral striatum. (b) Lentivirus-mediated knockdown of MeCP2 in the dorsal striatum blocks the development of escalated cocaine intake and reverses the long-term trajectory of cocaine-taking behavior in rats with extended access (Two-way ANOVA, Virus: _F_9,72=7.3, P<0.0001; Virus × Session: _F_1,8= 19.6, P=0.05). (c) MeCP2 knockdown flattens the cocaine dose-response curve in rats with extended cocaine access (Two-way ANOVA, Virus: _F_1,8=11.6, P<0.01; Dose: _F_4,32=4.7, P<0.005; Virus × Dose: _F_4,32= 6.7, P<0.005). (d) MeCP2 knockdown did not alter cocaine intake in rats with restricted access to the drug. In all cases, _n_=6 rats per group, and error bars are given as s.e.m.
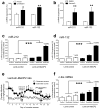
Figure 3. MeCP2 blunts the effects of cocaine on microRNA-212 expression
(a) Knockdown of MeCP2 in HEK-293 cells increases expression levels of miR-212 and miR-132. *P<0.05, **p<0.01, t-test compared with vector-transfected cells. (b) The DNA methyltransferase inhibitor 5-aza-dCincreases expression levels of miR-212 and miR-132 in HEK cells. **P<0.01, t-test compared with vector-transfected cells. (c) Lentivirus-mediated knockdown of MeCP2 expression in the dorsal striatum potentiates the stimulatory effects of cocaine on miR-212 expression (Two-way ANOVA, Virus: _F_1,12=607.0, P<0.0001; Access: _F_2,12=438.6, P<0.0001; Virus × Access: _F_2,12= 363.5, ***P<0.0001). (d) Knockdown of MeCP2 also potentiates the stimulatory effects of cocaine on miR-132 expression in dorsal striatum (Virus: _F_1,12=387.3, P<0.0001; Access: _F_2,12=263.3, P<0.0001; Virus × Access: _F_2,12= 198.9, ***P<0.0001). (e) Disruption of striatal miR-212 signaling using an antisense oligonucleotide (LNA-antimiR-212) “rescues” low levels of cocaine intake in Lenti-sh-MeCP2 rats with extended access. Striatal infusion of a control oligonucleotide(LNA-Scrambled) did not alter cocaine in restricted or extended access Lenti-sh-MeCP2 rats. *P<0.05, compared with intake on access day 9 (the session after the final LNA-antimiR-212 injection). (f) Lentivirus-mediated knockdown of MeCP2 in dorsal striatum potentiates the stimulatory effects of cocaine on the CREB-responsive gene c-fos (Virus: _F_1,12=20.9, P<0.0001, ***P<0.0001; Access: _F_2,12=4.4, P<0.05). In all cases, samples were run in triplicate for in vitro studies, there were _n_=6 rats per group for in vivo studies, and error bars are given as s.e.m.
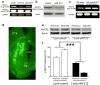
Figure 4. MicroRNA-212 represses MeCP2
(a) Using reverse transcription PCR and primers designed to selectively detect the long or short form of MeCP2, we found that miR-212 overexpression in rat PC12 cells resulted in a selective reduction in expression of the long MeCP2 transcript. (b) Representative immunoblot demonstrating that total protein levels of MeCP2 were decreased in PC12 cells following overexpression of miR-212. (c) LNA-antimiR-212 increased expression of the long MeCP2 transcript. (d) Representative GFP immunochemistry staining from the brain of a Lenti-miR-212 rat. (e) Representative immunoblot demonstrating dorsal striatal expression of MeCP2 in Lenti-control and Lenti-miR-212 rats with restricted or extended access to cocaine self-administration. (f) Relative amounts of MeCP2 in dorsal striatum was quantified by densitometry (Two-way ANOVA, Access: _F_1,18=219.4, P<0.0001; Virus × Access: _F_1,18= 75.9, ***P<0.0001). In all cases, samples were run in triplicate for in vitro studies, there were _n_=6 rats per group for in vivo studies, and error bars are given as s.e.m.
Figure 5. MeCP2-microRNA-212 interplay controls striatal BDNF expression
(a) BDNF expression is increased in the dorsal striatum of rats with extended access to cocaine self-administration. Upper panel, Representative immunoblot demonstrating dorsal striatal expression of BDNF in cocaine-naïve control rats, and in rats with restricted or extended access to cocaine self-administration. Lower panel, Relative amounts of BDNF in dorsal striatum was quantified by densitometry. *P<0.05, post-hoc comparison after significant one-way ANOVA. (b) Knockdown of MeCP2 in dorsal striatum decreases BDNF expression in cocaine self-administering rats. Upper panel, Representative immunoblot demonstrating dorsal striatal BDNF expression in Lenti-control and Lenti-sh-MeCP2 rats with restricted or extended access to cocaine self-administration. Lower panel, Relative amounts of BDNF in dorsal striatum was quantified by densitometry (Two-way ANOVA, Virus: _F_1,8=556.9, P<0.0001; Access: _F_1,8=97.0, P<0.0001; Virus × Dose: _F_1,8=24.2, **P=0.005). (c) Overexpression of miR-212 in dorsal striatum decreases BDNF expression in cocaine self-administering rats. Upper panel, Representative immunoblot demonstrating dorsal striatal BDNF expression in Lenti-control and Lenti-miR-212 rats with restricted or extended access to cocaine self-administration. Lower panel, Relative amounts of BDNF in dorsal striatum was quantified by densitometry (Two-way ANOVA, Access:_F_1,8=137.8, P<0.0001; Virus × Dose: _F_1,8=95.8, ***P=0.001). In all cases, _n_=6 rats per group and error bars are given as s.e.m.
Figure 6. Enhanced BDNF expression triggers compulsive cocaine intake
(a) Representative GFP immunochemistry staining from the brain of a Lenti-BDNF rat. (b) Striatal BDNF overexpression accelerates the development of escalated cocaine intake and precipitates a compulsive-like loss of control over intake (Two-way ANOVA, Virus: _F_1,8=28.1, P<0.0001; Session: _F_18,144=10.8, P<0.0001; Virus × Session: _F_18,144= 1.9, P=0.005). (c) BDNF overexpression shifts the cocaine dose-response curve upward in rats with extended access to the drug (Virus: _F_1,9=31.4, P<0.0005; Dose: _F_5,45=35.9, P<0.0001; Virus × Dose: _F_5,45= 2.87, P<0.05). (d) BDNF expression in the dorsal striatum in Lenti-control and Lenti-BDNF rats with restricted or extended access to the drug. Upper panel, Representative immunoblot demonstrating dorsal striatal BDNF expression in Lenti-control and Lenti-BDNF rats with restricted or extended access to cocaine self-administration. Lower panel, Relative amounts of BDNF in dorsal striatum was quantified by densitometry (Two-way ANOVA, Virus: _F_1,8=37.6, **P<0.0005; Access: _F_1,8=57.3, P<0.0001). In all cases, _n_=6 rats per group and error bars are given as s.e.m.
Figure 7. Disruption of endogenous BDNF transmission decreases cocaine intake
Disruption of endogenous BDNF signaling in dorsal striatum using an anti-BDNF neutralizing antibody decreases cocaine intake in rats with extended but not restricted access to cocaine. Control IgG infusions into the dorsal striatum did not alter cocaine in the restricted or extended access rats. Two-factor ANOVA of cocaine intake data on access days 20–25 was carried out: Access: _F_1,10=41.8, P<0.0001; Session: _F_5,50=3.5, P<0.01; *P<0.05, compared with intake on access day 20 (the session before the first anti-BDNF antibody infusion). There was an _n_=6 rats per group and error bars are given as s.e.m.
Comment in
- MeCP2 and drug addiction.
Feng J, Nestler EJ. Feng J, et al. Nat Neurosci. 2010 Sep;13(9):1039-41. doi: 10.1038/nn0910-1039. Nat Neurosci. 2010. PMID: 20740030 - Addiction: Cracking the code of addiction.
Welberg L. Welberg L. Nat Rev Neurosci. 2010 Oct;11(10):668. doi: 10.1038/nrn2921. Nat Rev Neurosci. 2010. PMID: 21080538 No abstract available.
Similar articles
- Increased cocaine-induced conditioned place preference during periadolescence in maternally separated male BALB/c mice: the role of cortical BDNF, microRNA-212, and MeCP2.
Viola TW, Wearick-Silva LE, De Azeredo LA, Centeno-Silva A, Murphy C, Marshall P, Li X, Singewald N, Garcia F, Bredy TW, Grassi-Oliveira R. Viola TW, et al. Psychopharmacology (Berl). 2016 Sep;233(17):3279-88. doi: 10.1007/s00213-016-4373-z. Epub 2016 Jul 9. Psychopharmacology (Berl). 2016. PMID: 27392631 - MeCP2 controls hippocampal brain-derived neurotrophic factor expression via homeostatic interactions with microRNA‑132 in rats with depression.
Su M, Hong J, Zhao Y, Liu S, Xue X. Su M, et al. Mol Med Rep. 2015 Oct;12(4):5399-406. doi: 10.3892/mmr.2015.4104. Epub 2015 Jul 20. Mol Med Rep. 2015. PMID: 26239616 - MeCP2 and drug addiction.
Feng J, Nestler EJ. Feng J, et al. Nat Neurosci. 2010 Sep;13(9):1039-41. doi: 10.1038/nn0910-1039. Nat Neurosci. 2010. PMID: 20740030 - MeCP2 and the enigmatic organization of brain chromatin. Implications for depression and cocaine addiction.
Ausió J. Ausió J. Clin Epigenetics. 2016 May 21;8:58. doi: 10.1186/s13148-016-0214-5. eCollection 2016. Clin Epigenetics. 2016. PMID: 27213019 Free PMC article. Review. - The ups and downs of BDNF in Rett syndrome.
Sun YE, Wu H. Sun YE, et al. Neuron. 2006 Feb 2;49(3):321-3. doi: 10.1016/j.neuron.2006.01.014. Neuron. 2006. PMID: 16446133 Review.
Cited by
- Alteration of twinfilin1 expression underlies opioid withdrawal-induced remodeling of actin cytoskeleton at synapses and formation of aversive memory.
Wang YJ, Yu C, Wu WW, Ju YY, Liu Y, Xu C, Long JD, Zan GY, Wei XY, Zhang LS, Chai JR, Chen Z, Liu JG. Wang YJ, et al. Mol Psychiatry. 2021 Nov;26(11):6218-6236. doi: 10.1038/s41380-021-01111-3. Epub 2021 May 7. Mol Psychiatry. 2021. PMID: 33963280 - Cocaine-regulated microRNA miR-124 controls poly (ADP-ribose) polymerase-1 expression in neuronal cells.
Dash S, Balasubramaniam M, Martínez-Rivera FJ, Godino A, Peck EG, Patnaik S, Suar M, Calipari ES, Nestler EJ, Villalta F, Dash C, Pandhare J. Dash S, et al. Sci Rep. 2020 Jul 8;10(1):11197. doi: 10.1038/s41598-020-68144-6. Sci Rep. 2020. PMID: 32641757 Free PMC article. - Epigenetics of drug abuse: predisposition or response.
Nielsen DA, Utrankar A, Reyes JA, Simons DD, Kosten TR. Nielsen DA, et al. Pharmacogenomics. 2012 Jul;13(10):1149-60. doi: 10.2217/pgs.12.94. Pharmacogenomics. 2012. PMID: 22909205 Free PMC article. Review. - Interactions between Early Life Stress, Nucleus Accumbens MeCP2 Expression, and Methamphetamine Self-Administration in Male Rats.
Lewis CR, Bastle RM, Manning TB, Himes SM, Fennig P, Conrad PR, Colwell J, Pagni BA, Hess LA, Matekel CG, Newbern JM, Olive MF. Lewis CR, et al. Neuropsychopharmacology. 2016 Nov;41(12):2851-2861. doi: 10.1038/npp.2016.96. Epub 2016 Jun 17. Neuropsychopharmacology. 2016. PMID: 27312406 Free PMC article. - Nanoparticle delivery systems for substance use disorder.
Kasina V, Mownn RJ, Bahal R, Sartor GC. Kasina V, et al. Neuropsychopharmacology. 2022 Jul;47(8):1431-1439. doi: 10.1038/s41386-022-01311-7. Epub 2022 Mar 28. Neuropsychopharmacology. 2022. PMID: 35351961 Free PMC article. Review.
References
- Nan X, et al. Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature. 1998;393:386–389. - PubMed
- Amir RE, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–188. - PubMed
- Cassel S, et al. Fluoxetine and cocaine induce the epigenetic factors MeCP2 and MBD1 in adult rat brain. Mol Pharmacol. 2006;70:487–492. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases